OZONE
Ozone is one of those pollutants we often hear about, but what exactly is it? Well, ozone plays two very different roles in our environment. High up in the atmosphere, it forms a protective layer that shields us from the sun’s harmful ultraviolet rays, almost like a natural sunscreen for the Earth. But it’s a different story when ozone is closer to the ground. It becomes a pollutant that can harm our lungs and damage plants. A good way to remember ozone’s role in the stratosphere vs. troposphere is “ozone is good up high and bad nearby.”
This makes ozone both a good and a bad pollutant, depending on where it is. Understanding how ozone works is really important because it has a big impact on our environment and our health. So, let’s look closer at ozone and see why it’s such an important part of our world. In this article, we’ll dive into everything you need to know about ozone. We’ll talk about where it comes from, what levels are safe in the air around us, and how it affects our health and the environment. We’ll also look at what can be done to manage ozone levels, why monitoring it is so important, and how we can keep track of it.
1. What is O3?
Ozone (O3) is made up of three oxygen atoms. It exists naturally. The most abundant gas in the atmosphere is nitrogen (78%), followed by oxygen (21%). The remaining 1% is a collection of many other gases. Examples include carbon dioxide, neon, and hydrogen. Ozone is also one of the gases represented by this 1% proportion. It amounts to approximately 0.00001% of ozone in the ambient air. The US EPA has defined six criteria for air pollutants, including ozone (O3), which is extensively monitored by regulatory authorities worldwide.
It is a pale blue gas with a distinctively pungent, irritating smell resembling chlorine bleach and can be detectable at 0.1 ppm concentrations in the air. Being a powerful oxidizing agent, it is highly combustible at 10wt% or higher concentrations. Also, it is highly unstable and hence gets converted to O2 at high concentrations.
Ozone in Atmosphere:
Ozone in the atmosphere is a topic that plays a crucial role in understanding our planet’s health. Ozone exists in both the Earth’s upper atmosphere and at ground level. Ozone can be beneficial or harmful depending on where it is found. It is both a natural and a man-made product that occurs in the Earth’s upper atmosphere ozone molecule (the stratosphere) and lower atmosphere (the troposphere).
Stratospheric Ozone
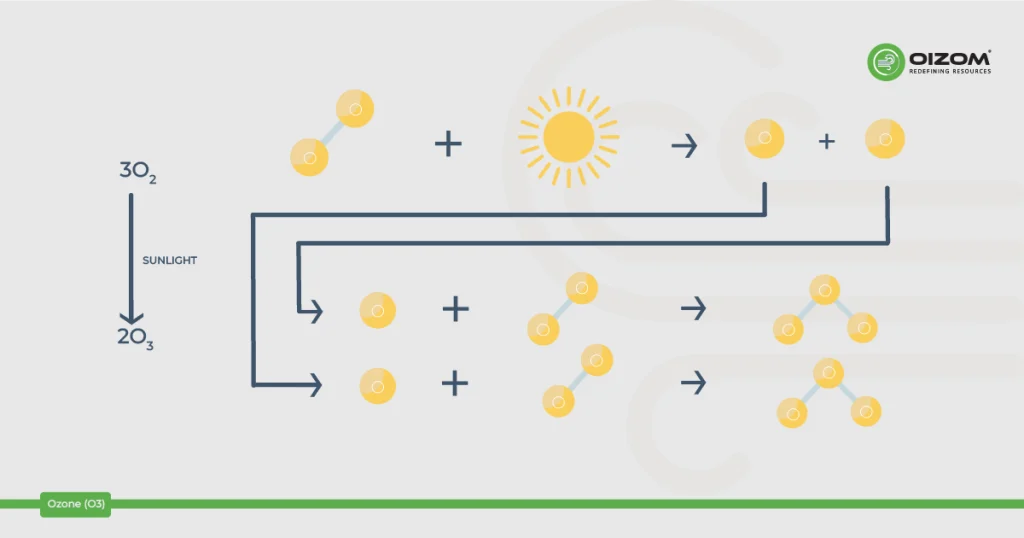
- Stratospheric ozone is generated naturally by interacting solar ultraviolet (UV) light with molecular oxygen (O2). The “ozone layer,” which extends 10 to 50 kilometers above the Earth’s surface, decreases the quantity of dangerous UV light that reaches the surface. It acts as our natural sunscreen.
- It absorbs the sun’s damaging ultraviolet radiation, preventing them from reaching the surface or the air. This layer is being depleted due to humans’ continued use of CFCs.
- Did you know that CFCs were once commonly used in products like aerosol sprays, foams, packaging materials, solvents, and refrigerators? However, they were found to be a major cause of ozone layer depletion, leading to a large ozone hole over Antarctica every spring. Their production for these uses was banned in 2010 under the Montreal Protocol. (Source – https://bit.ly/41j0HL9)
- Because CFCs are highly stable, they can remain in the environment for a very long time after being discharged (10–100 years).
Tropospheric or Ground-level ozone
- Tropospheric ozone (O3) or ground-level ozone is a gas found in the lowest layer of the Earth’s atmosphere, the troposphere, which extends up to 10 kilometers.
- What we breathe is mostly created by photochemical interactions between two major types of air pollutants: volatile organic compounds (VOC) and nitrogen oxides (NOx). These reactions have traditionally depended on heat and sunlight, resulting in higher ambient ozone concentrations in the daytime.
- When ozone gets trapped indoors, it can irritate the respiratory system, especially for people with asthma, and may even harm plants. Without fresh outdoor air, ozone levels indoors naturally drop to nearly zero, creating ideal conditions for mold, bacteria, and viruses to grow. To maintain good air quality and prevent these issues, it’s important to keep indoor ozone levels within a safe range, as poor air quality can lead to health problems and discomfort.
Did you know this? Ozone has a half-life of six minutes in an office environment. It is a highly toxic gas and is the most serious health risk from photocopiers.
2. Sources of O3
Ground-level ozone is formed in the atmosphere from gases emitted by tailpipes, smokestacks, industries, and other pollution sources. When these gases come into contact with sunlight, they react, producing ozone pollution.
Ambient Source: Many people think high ozone levels only happen in big cities, but that’s not true. Ozone can form anywhere, including smaller cities. It’s not just a local problem either; ozone and its Contributors, VOCs (volatile organic compounds) and NOx (nitrogen oxides), can travel hundreds of miles, affecting air quality in urban and rural areas. Ozone levels are usually highest in the afternoon when sunlight is strongest. Still, areas that are downwind of pollution sources can see ozone peaks later in the day or even at night as winds carry it far from where it was created. This means high ozone levels can happen in remote areas and at different times of day.
You might have noticed that “ozone action day” warnings often advise against activities like mowing your lawn or driving your car. This is because exhaust from lawnmowers and gasoline vapors release NOx and VOCs, gases that combine with heat and sunlight to form ozone.
Additionally, it also has indoor sources. These include:
- Photocopiers: Ozone emissions from photocopying machines have been discovered as indoor air pollution hazardous to workers and clients that are very close to the machine during operation. A plain paper photocopier produces an image by reflecting light from the original object onto an electrically charged drum or belt. During this process, pollutants such as ozone, hydrocarbons, VOCs, and dust are emitted, posing an environmental risk. Photocopying releases the major pollutant, Ozone (O3), an unstable form of oxygen.
- Some Air-cleaning Systems can produce O3, which can harm one’s health. A few air purifiers purposely emit large levels of O3, the primary cause of smog.
- UV Lights or Lamps: UV Light ranging from 160 to 240 nm can split O2 molecules into two atoms, resulting in O3. They then bind to other O2 molecules. And it produces ozone (O3).
- Home Electrical Appliances: Many household equipment, including refrigerators, air conditioners, vegetable washers, and facial steamers, include built-in ozone producers, commonly known as ionizers.
- Disinfectants: Ozone has a high oxidizing power. As a result, it is used in a variety of sterilizing and disinfecting agents commonly found in hospitals. However, these disinfectants generate ozone gas, which, if trapped, might be harmful.
High ozone concentrations are found to be downwind of NOx and VOC sources in urban areas, as pollutants take a while to react with sunlight. Peak concentration usually occurs during the afternoon hours when there is more sunlight. However, peaks in the afternoon and evening are observed in the areas downwind of the major sources as its precursors are long distances by the wind. Thus, in remote areas, high ozone levels can occur at various times of day, including early evening or night.
3. Permissible Levels of O3
Several countries have established regulations to limit the amount of tropospheric ozone in the open air. These laws establish restrictions reflected in the Air Quality Index (AQI), which includes total ozone, moderate ozone, and other contaminants. Thresholds vary depending on geographic location, season, and time of day.
The breakpoint concentrations describing air quality based on ground-level ozone concentrations for different countries are here. In India, the daily O3 levels of up to 100 µg/m3 are considered satisfactory.
Table: Breakpoints of Ozone (µg/m3)
|
India (8-hr) |
US (8-hr) |
China (8-hr) |
EU (8-hr) |
||||
|
AQI Category |
Breakpoint concentration |
AQI Category |
Breakpoint concentration |
AQI Category |
Breakpoint concentration |
AQI Category |
Breakpoint concentration |
|
Good |
50 |
Good |
100 |
Excellent |
116 |
Very low |
60 |
|
Satisfactory |
100 |
Moderate |
160 |
Good |
147 |
Low |
120 |
|
Moderately polluted |
200 |
Unhealthy for sensitive |
215 |
Lightly Polluted |
186 |
Medium |
180 |
|
Poor |
265 |
Unhealthy |
265 |
Moderately Polluted |
225 |
High |
240 |
|
Very Poor |
748 |
Very Unhealthy |
800 |
Heavily Polluted |
733 |
Very high |
240+ |
|
Severe |
748+ |
Hazardous |
– |
Severely Polluted |
– |
||
The Clean Air Act governs most tropospheric ozone regulations in the United States. The United States Environmental Protection Agency (EPA) establishes national ozone air quality guidelines to safeguard public health and the environment.
The European Union regulates ozone in ambient air under Directive 2002/3/EC of the European Parliament and the Council. Ensuring good protection against the health risks associated with ozone exposure is critical. The negative effects of ozone on flora, ecosystems, and the environment should be minimized as much as possible. Ozone pollution’s transboundary nature necessitates community-wide action.
Globally, the World Health Organization (WHO) recommends maximum ground-level ozone concentration levels. The WHO’s 2021 report states that they recommend a maximum ozone level of 100 µg/m3 based on 8-hour averaging times, or the equivalent of roughly 47 parts per billion (ppb)
4. Health & Environmental Impact of O3
Health Impact
Anyone who spends time outside in areas with high ozone pollution levels may be in danger. Exposure to ozone can have varying effects on your health. For example, the hazards increase if ozone levels rise, if you breathe faster when working or exercising outside, or if you spend more time outside.
When exposed to O3, it can cause a wide range of illnesses and discomforts. Ozone at high concentrations can irritate the skin and cause cellular damage. Ozone can have a variety of health consequences on the human body.
Ozone can irritate the eyes, nose, and throat and aggravate asthma and other lung diseases, including bronchitis, heart disease, reduced lung capacity, etc. Ozone can irritate the respiratory system, causing coughing and an uncomfortable sensation in the chest. However, ozone can continue harming your lungs if levels remain high after symptoms disappear.
Individuals already suffering from heart or lung disease when exposed to high ozone levels can increase their risk of premature death. Children, especially those who spend much time outdoors, are at particular risk under high ozone concentrations.
Were you aware of this? Almost one in four people in the US is exposed to harmful air pollution. The American Lung Association revealed that 131 million people, more than one-third of the US, are exposed to unhealthy ozone and particle pollution. Following more stringent federal standards for particle pollution, this total figure represents an 11.7 million increase from the previous year.
Environmental Impact
Ozone exposure decreases plant productivity by harming cells and destroying leaf tissue. As a result, ozone depletes plants’ photosynthesis capacity and produces food. It also reduces crop and timber yields, resulting in millions of dollars in economic losses. Were you aware of this? One study found between 2% and 14% decreases in yields for maize, wheat, and soybean globally attributable to ground-level ozone pollution.
The photolysis of ozone by UV light leads to removing hydrocarbons from the air. Still, it also forms components of smog (i.e., fog or haze intensified by smoke or other air pollutants), such as peroxyacetyl nitrates (PAN), which can be powerful eye irritants. UV rays have a significant impact on plankton. These are higher up the aquatic food chain. Plankton destruction affects all organisms in the food chain.
5. Possible Corrective Measures
The primary step is O3 monitoring to identify the areas with high O3 levels or areas where air quality does not meet the O3 national standards. In addition to this, the following corrective measures can be taken:
- More stringent measures implemented nationwide under the federal Clean Air Act have helped to reduce pollutants that lead to ozone formation. Power plants, industrial sites, and on-road vehicles are cleaner than they used to be, resulting in a nationwide improvement in air quality.
- Individuals can take steps to protect themselves on days with unhealthy levels of air pollutants and ask policymakers at all levels of government to continue to require the cleanup of air pollution.
- Have a walk, use bicycles, public transport, or carpool whenever possible.
- Refuel your vehicle after dusk and avoid overflowing your tank.
- Avoid keeping your vehicle idle for long.
- Promote alternative fuels and improve quality fuels with high emission rates.
- Periodically maintain/service your vehicles to improve their performance and reduce emissions.
- Avoid unnecessary use of paints or other products that release the solvent.
- Also, avoid physical activity outside when pollution levels are high, especially on hot days. Stay indoors until it cools down outside and the air is fresh.
6. Different Working Principles for O3 Monitoring
Different working principles for ozone monitoring in the ambient environment are U.V. Photometry, Gas-Phase Chemiluminescence, semiconductor, and electrochemistry.
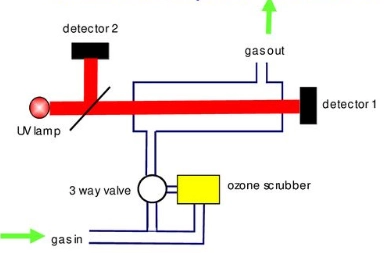
U.V. Photometry: The air sample taken by the ozone monitor is divided into two parts. From one part of the sample, O3 is removed from the air using a scrubber to provide zero reference intensity. The air sample in the O3 monitor working on the principle of U.V. Photometry is exposed to ultraviolet (UV) light at 254 nm wavelength, where the ozone present in the air sample absorbs the UV light in proportion to its concentration. The absorption is measured using a UV detector, and the ozone concentration is calculated as opposed to the sample without ozone. It is the most widely used conventional method for measuring O3 concentrations.
[Source: https://slideplayer.com/slide/14795597/]
Gas-Phase Chemiluminescence: It is an old method for ozone monitoring. Here, the ambient air and ethylene are directly sent together in a mixing zone of the O3 monitor, where ozone in the air reacts with ethylene. As this reaction is photolytic, it emits light proportional to the O3 concentration in the air sample, which a photomultiplier tube detects. The photocurrent that is measured is amplified and converted to O3 concentration values.
Semiconductor – When a metal oxide semiconductor-based O3 monitor is exposed to an air sample, the ozone molecules react on the metal oxide surface of the sensor and dissociate into charged ions that alter the film’s resistance. This interaction is measured as a signal and is converted to the respective gas concentration. However, the energy consumption of such ozone monitors is higher than that of other O3 monitors.
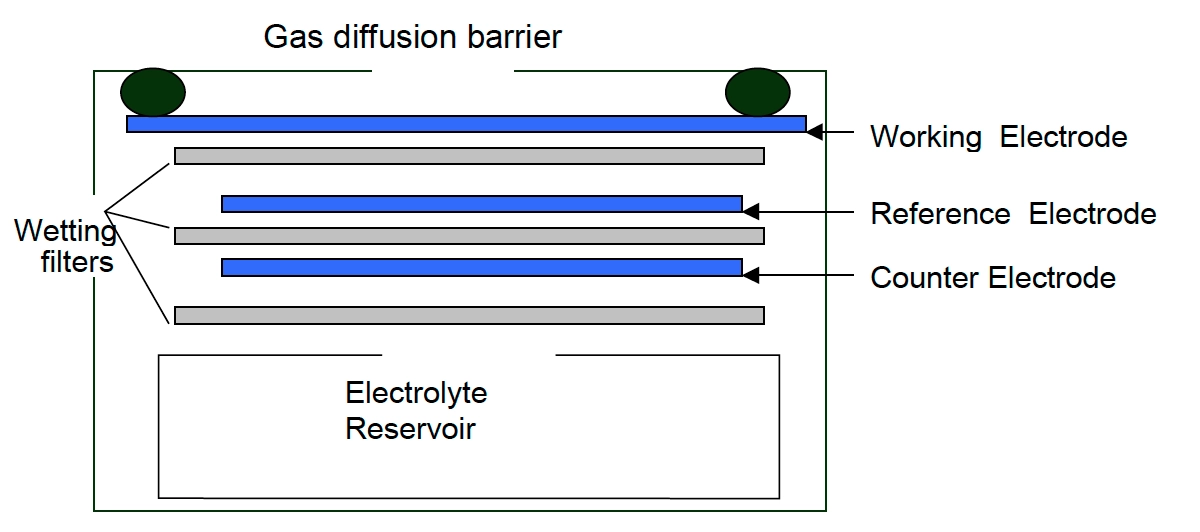
Electro-chemical- Ozone monitors working on the electrochemical principle are operated based on the diffusion of ozone gas into the sensor, producing an electrical signal proportional to the O3 concentration. It allows accurate measurement of even low concentrations of ground-level ozone, essential in O3 monitoring in the ambient air.
[Source: iSCAPE Sensors Documentation]
7. Oizom’s Sensor Working Principle for O3 Monitoring
Oizom provides a range of Ozone (O3) sensor modules to monitor varying O3 levels based on your needs. Our sensors accurately measure O3 in ambient conditions, detecting concentrations in ppb/ppm. This sensor monitors ozone in real-time. This sensor is integrated into a metal casing and ultra-low-noise support electronics, making it compact and reliable. This allows accurate gas detection even at very low concentrations in the atmosphere. This sensor works on the Electrochemical working principle to measure environmental air quality.
This sensor undergoes calibration using standard gases and tools to ensure high sensitivity and accuracy. Each gas sensor is calibrated with zero and span checks in a controlled lab, following Section 12.2 of the USEPA Quality Assurance Handbook for Air Pollution Measurement Systems Volume II. Calibration is done using NIST-traceable gas standards for reliable performance.
This O3 sensor module is utilized in Oizom’s Polludrone and AQBot systems. It’s perfect for applications like smart city initiatives, smart campuses, airport monitoring, roadside monitoring, research projects, and environmental impact assessments. By utilizing this sensor module, users can ensure they receive accurate, real-time data on O3 levels, aiding in effectively monitoring and managing air quality across diverse environments.
8. Why Choose Oizom O3 Sensor?
- Compact: Our sensors are small and easy to install, perfect for use in any space, making them ideal for portable air quality monitoring. The O3 sensors come pre-calibrated and can be quickly replaced in just a few minutes by removing and replacing the old sensor with a new one.
- Durable: The O3 sensor has a long life of almost two years.
- Energy Efficient: There is no need to be concerned about energy usage because our sensors are accurate and energy efficient. Powered up with just 3.3 to 5V for efficient, reliable performance!
- In-house sensor tech: Oizom applies advanced data processing algorithms to compensate for the effects of temperature and humidity on the sensor output. The algorithm is designed to automatically update based on weather conditions and seasonal changes, removing its influence on the sensor performance. The advanced algorithms also compensate for the effect of the cross-sensitive gas.
- Ultra-Low Noise Electronics: The sensor module’s design is crucial for accurate measurements. Each sensor is housed in a metal casing with ultra-low-noise electronics and a base PCB. The metal casing shields the sensor and electronics from electromagnetic interference, preventing false readings.
- RoHS Compliant: Our sensors comply with the RoHS criteria for restricting hazardous substances in electrical and electronic devices.
9. Reasons Why O3 Monitoring is Important
- Ground-level ozone is one of the critical air pollutants produced indirectly from various common sources, such as vehicular emissions and energy production, resulting in poor air quality in urban and rural areas.
- O3, apart from affecting human health and the environment, plays a major role in the formation of smog, which harms health and the environment.
- High levels of O3 harm lung tissue, and continued exposure can affect the respiratory system long after symptoms disappear. High ozone levels inhibit plant growth, resulting in decreased agricultural productivity.
- O3 monitoring is an efficient way to detect the accumulation of high levels of O3 we are exposed to and alerts us when a certain level is exceeded.
- Real-time monitoring of O3 levels helps calculate the air quality index to deliver health advisories and formulate an action plan to meet standards.
- Raising knowledge about the effects of ozone and other air pollutants is a crucial step toward achieving favorable environmental outcomes. Let me share a fact: A study conducted as part of the project, which installed ozone and weather monitoring stations in over 100 schools worldwide, found that approximately 43% of the middle and high school students surveyed had no prior knowledge of ground-level ozone. Still, by the end of the project, 98% of students reported being familiar with the pollutant.
FAQs
- What is ozone (O3)?
Ozone (O3) is a gas found in the Earth’s upper atmosphere and ground level. In the upper atmosphere, it protects us from harmful UV rays, but it’s a harmful pollutant at ground level. - How does ground-level ozone affect human health?
Ground-level ozone can cause respiratory issues like coughing, throat irritation, and worsened asthma. Prolonged exposure may harm lung function.
- Where does ground-level ozone come from?
It forms when pollutants from vehicles, factories, and other sources react with sunlight, creating smog in urban areas.
- Is ozone the same in the upper atmosphere and at ground level?
No. In the upper atmosphere, ozone forms a protective layer against UV radiation, but it’s a harmful air pollutant at ground level.