Summary
Hydrogen plays a key role in clean energy as it is lightweight, abundant, and zero-carbon at the point of use. But here lies the twist: when hydrogen leaks, it is not so innocent. Hydrogen does not retain heat per se, but alters the atmospheric composition in a way that allows methane, tropospheric ozone, and stratospheric water vapor to trap even more heat overall. Over a 100-year time period, 1 kg of hydrogen leakage can cause warming equivalent to approximately 12 kg of CO2.
But the good news is that we can be proactive with hydrogen; smart design, compatible materials, preventative monitoring, good maintenance, and diligent reporting are all methods one can use to mitigate the issue. Even without mentioning vapor, even small leaks of about 1,000 units can add up globally. To conclude, hydrogen can be a local or even global climate friend, but only if it is produced sustainably and used effectively.
Hydrogen Gas: Not Just Clean Energy, But a Climate Player
Hydrogen is usually seen as a clean fuel of the future – light-weight, readily available, and ideal for a zero-carbon world. However, this “ideal” molecule has a sneaky side, as it escapes into the atmosphere, it does not trap heat directly like atmospheric CO₂ or methane. Instead, hydrogen triggers the key chemical reactions that enhance the effect of other greenhouse gases, subsequently warming the planet.
Scientists have captured this phenomenon in the form of Global Warming Potential (GWP100), where a slight leakage of hydrogen can have amplified effects on methane, tropospheric ozone, and stratospheric water vapor, as even a small amount of hydrogen would release energy into the atmosphere.
This blog explores how hydrogen leakage works, its climate implications, and strategies to mitigate its effects, explained with scientific insight.
Global Warming Potential of Hydrogen
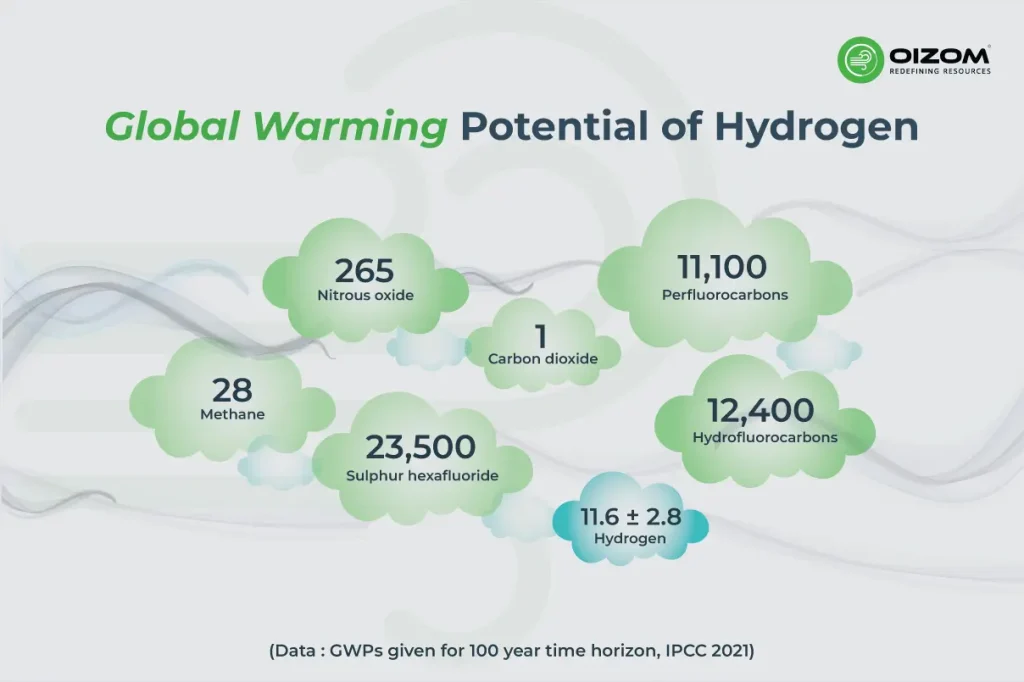
When scientists assess the climate impacts of any gas, they utilize a metric known as Global Warming Potential (GWP) to show how much heating a gas incurs relative to carbon dioxide (CO₂). GWP over 100 years (GWP100) is the most frequently used metric.
However, there is an important distinction: Hydrogen (H₂) is not a greenhouse gas like CO₂, methane, or nitrous oxide, as it does not absorb infrared radiation. Instead, hydrogen alters the chemistry of the atmosphere in ways that increase the abundance of greenhouse gases. Hydrogen in the troposphere reacts with the hydroxyl radical (OH), the agent responsible for breaking down methane. When hydrogen is present, there will be less OH, slowing methane’s natural breakdown, which leads to elevated methane concentrations and stronger warming. This shift in chemistry will also affect the atmospheric concentrations of other gases that impact climate:
Tropospheric ozone (O₃) is likely to increase, which contributes to short-term warming. Additionally, stratospheric water vapor (H₂O) will rise, retaining more heat at higher altitudes.
Thus, hydrogen can be considered a short-lived yet significant contributor to warming. On that note, recent global model studies have estimated that the leakage of 1 kg of hydrogen has over 11-12 times the GWP warming impact of 1 kg of CO₂ over 100 years (GWP).
Key Numbers at a Glance
The following table provides the Global Warming Potential (GWP) values and the atmospheric lifetime of hydrogen (H₂) from the 2023 multi-model study led by Maria Sand et al. in Communications Earth & Environment.
These estimates come from a set of five global atmospheric chemistry models that estimated hydrogen’s impact on methane, ozone, and stratospheric water vapor.
All numbers reported are rounded for easy reading, and the referred ranges include reports of likely uncertainty.
| Metric | Value | Notes |
|---|---|---|
| GWP₂₀ | ~37 (±15) | Short-term climate impact is higher because methane dominates early warming. |
| GWP₁₀₀ | ~11.6 (±2.8) | Benchmark value used for long-term planning and policy comparison. |
| GWP₅₀₀ | ~3.3 (±1.0) | Long-horizon influence decreases as short-lived effects decay. |
| Hydrogen atmospheric lifetime | ~2 years (1.9 – 2.7 yrs model range) | Indicates hydrogen is a short-lived climate forcer, not a persistent gas like CO₂. |
Overall, the fact that hydrogen is present in the atmosphere for only two years makes its indirect chemical effects highly warming-intensive compared to CO₂ in the short- to medium-term.
On the order of a 100-year timescale, one kilogram of leaked hydrogen warms roughly twelve times as much as a kilogram of CO₂ warming, and this shows the importance of even small leaks toward climate objectives.
How Leaked Hydrogen adds to Warming
The climatic impact of hydrogen comes not from the gas itself, but from the chemical sequence that follows a leak into the air. Hydrogen doesn’t change the atmosphere, but instead interacts with the hydroxyl radical (OH), the molecule that typically removes greenhouse gases from the atmosphere, such as methane (CH₄). This sequence of events involves a domino effect, altering concentrations of methane, ozone, and stratospheric water vapor, all of which are short-lived climate forcers that together increase the planet’s temperature.
Methane Lifetime Effect
Under normal conditions, OH radicals react with methane to begin its breakdown in the atmosphere. The main reaction series looks like this:
Initial reaction:
CH₄ + OH → CH₃ + H₂O
Subsequent steps:
CH₃ + O₂ → CH₃O₂ (methylperoxy radical)
→ Formation of formaldehyde (HCHO)
→ HCHO → CO + H₂O (oxidation step)
In some situations, OH radicals are produced again, allowing the cycle to continue and preventing changes in methane concentrations through the action of OH radicals. But upon gaseous hydrogen leakage, hydrogen competes with methane to react with OH. The reaction of hydrogen with OH reduces the clean-up capacity of the atmosphere:
H₂ + OH → H₂O + H·
This individual reaction leaves less OH, which can break down methane, leading to a longer life in the atmosphere.
Model simulations from Sand et al. (2023) produce compelling evidence that a 10% increase in hydrogen concentration leads to a pronounced decrease in OH with a parallel increase in the lifetime and abundance of methane. The outcome from more hydrogen is a more potent greenhouse effect, with methane accounting for around 44% of the total warming from hydrogen (GWP₁₀₀ ≈ 11.6 ± 2.8).
Ozone Formation
The alteration of atmospheric chemistry from hydrogen is not limited to only methane. As OH declines and methane rises, tropospheric ozone (O₃) production increases. This happens because the reaction of methane in the atmosphere produces a variety of intermediate compounds (such as CH₃O₂ and HO₂) that react with nitrogen oxides (NOₓ) in the presence of sunlight to form ozone. Ozone in the lower atmosphere is both a pollutant and a greenhouse gas. It absorbs infrared radiation and contributes to warming, particularly near the surface. Models from the multi-model study showed that the contribution of changes in ozone accounted for approximately 38% of hydrogen’s total warming due to indirect effects.
Stratospheric Water Vapor
Further up in the dry stratosphere, hydrogen oxidation contributes to the production of extra water vapor (H₂O). Although the stratosphere contains much less water vapor than the troposphere, this layer plays an outsized role in trapping heat, as stratospheric water vapor is long-lived and radiatively active. Using the models in the 2023 study, estimates indicated that this process contributes about 18% of hydrogen’s overall warming effect. Additional stratospheric water vapor will also cool the stratosphere slightly, altering the thermal balance and further influencing ozone chemistry.
What Do Leakage Rates Mean In Co₂e
Evaluating hydrogen leakage in carbon dioxide equivalents (CO₂e) enables the translation of complex chemistry into our understanding and real-world climate impacts.
Using the Global Warming Potential over 100 years, one kilogram of leaked hydrogen results in about twelve times more warming for the planet than one kilogram of CO₂ over 100 years.
How the Conversion Works
To estimate the climate cost of hydrogen leaks, multiply the amount of hydrogen released by its GWP value:
CO₂e = H₂ leaked × GWP₁₀₀
Example: 1 t of H₂ × 11.6 = 11.6 t CO₂e
The table below shows how different leakage rates translate into CO₂e for a facility handling 10,000 tonnes of hydrogen per year.
| Annual H₂ throughput | Assumed leak rate | H₂ leaked | CO₂e at GWP₁₀₀ (~11.6) |
|---|---|---|---|
| 10,000 t | 0.5 % | 50 t | ≈ 600 t CO₂e |
| 10,000 t | 1 % | 100 t | ≈ 1200 t CO₂e |
| 10,000 t | 3 % | 300 t | ≈ 3600 t CO₂e |
Even seemingly small leak rates add up. For large-scale hydrogen hubs or national infrastructure, a few percent loss could equate to thousands of tonnes of CO₂e emissions every year.
The Source Matters: Green vs. Fossil-Based Hydrogen
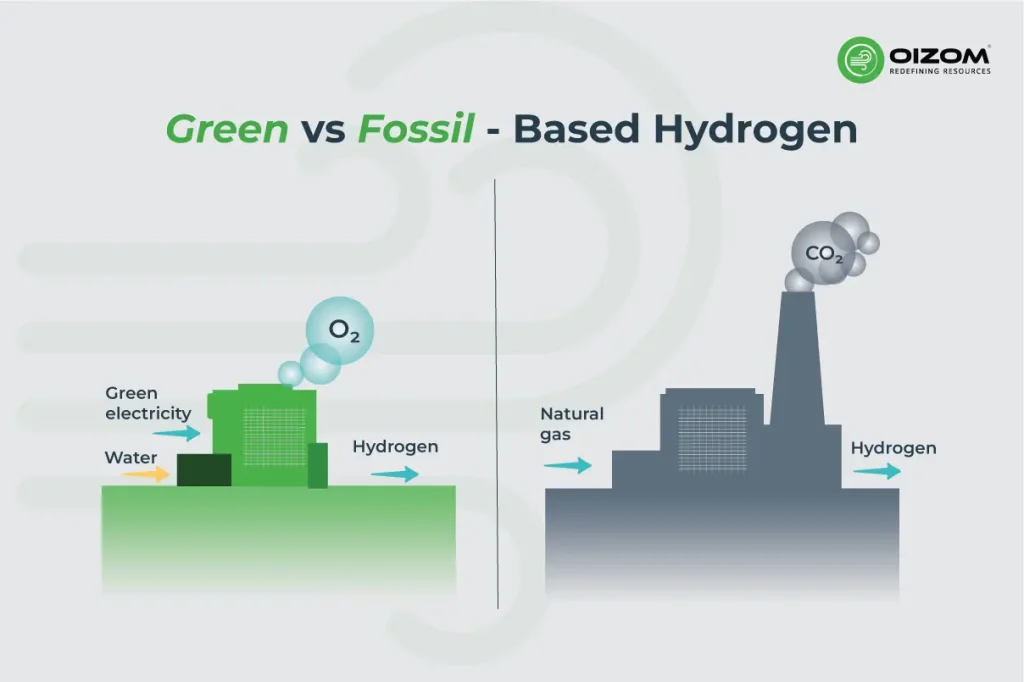
Hydrogen has different types as well, and not all hydrogen types carry the same climate footprint. “Green hydrogen,” made by splitting water using renewable electricity, can approach near-zero emissions if the process and power are clean. In contrast, “grey” or “blue” hydrogen produced from natural gas, often with incomplete carbon capture, still emits significant CO₂ and methane upstream. When leakage is added to that equation, the entire warming effect of fossil-based hydrogen can equate or even exceed that of the fuel it aims to replace. Therefore, both the production pathway and leakage control determine whether hydrogen truly acts as a climate solution or merely shifts the problem elsewhere.
Short-term vs. Long-term Impact
If we were to take this same calculation and change this to the 20-year GWP (≈ 37) instead of 11.6, the CO₂e values would triple, once again reflecting the higher short-term warming potential of hydrogen.
This further highlights why hydrogen poses both a long-term and short-term climate problem, and that minimizing leakage can yield immediate benefits.
Uncertainty and What To Watch
One of the most significant sources of uncertainty comes from the uptake of hydrogen by microbes in soils. While soils will generally consume hydrogen, the rate of uptake for a particular type of soil and moisture and temperature conditions is not easily determined or quantified.
Less significant factors include atmospheric OH radicals and the reference value used in the CO₂ to calculate GWP100, which is based on a CO₂ molecule and the reaction to which it is less than that of soil uptake in the atmosphere.
In practical terms, estimating hydrogen’s leakage by considering or modeling various values is preferable to relying on a single estimate of leakage quantity. Maintaining a margin of safety in emission targets helps ensure that strategies remain effective, even if actual atmospheric responses differ from initial assumptions.
Monitoring and Mitigation Strategy
Design to Mitigate Leakage
Mitigating hydrogen leaks starts at the design stage. Utilize materials compatible with hydrogen to prevent hydrogen embrittlement, and ensure all seals and fittings are rated for H₂ service. Limit the number of joints in a system, properly manage the pressure, and follow industry specifications for torque and pressure ratings to limit the risk of leakage.
Ongoing Monitoring and LDAR
Ongoing detection of hydrogen loss is most important. Use fixed sensors, portable sniffers, and mass balance calculations to detect the ongoing process of hydrogen loss. Perform phase surveys, log the data, and automate alerts to capture leaks and maintain a safe method promptly.
Operations and Maintenance
All routine operations and maintenance practices contribute to conditions that help prevent leakage. This includes commissioning checks, verifying torque testing conditions, regular maintenance of valve stem packing, following regular replacement intervals, and training staff on best practices for working with hydrogen systems, and thus adhering to the safety standards.
Reporting and Verification
Accurate reporting ensures accountability and compliance. Measurement-based, if possible, and conservative, if not, can be key factors; including some sort of periodic independent verification can reinforce reporting accuracy. Reports should be transparent and include continuous improvement in the safe management of hydrogen gas.
Conclusion
Hydrogen offers unparalleled potential for a cleaner future, but optimal climate benefits are predicated upon careful management. Even minor leaks can cause subtle changes in atmospheric chemistry that amplify warming. This is why careful design, monitoring, and maintenance are so important. With cautious storage of hydrogen, along with accurate tracking from production to use, we can ensure that this lightest of fuels delivers on its heavy-lifting climate promise.







