Methane (CH₄) is a strong greenhouse gas that plays a significant role in accelerating climate change. While it’s naturally produced through processes like the decomposition of organic matter in wetlands and the digestive systems of livestock, human activities have significantly increased methane levels in the atmosphere. Key contributors include agriculture, fossil fuel extraction, and waste management practices.
Methane is a combustible gas with a low Lower Explosive Limit (LEL) of 4.4%, but its impact on global warming is receiving more attention. CH4 monitoring is an efficient way to detect the buildup of CH4 levels and take necessary actions. This article covers information on methane gas, its sources in the ambient air, permissible levels, health and environmental impact, possible corrective measures, need for methane monitors as well as different methods of CH4 monitoring.
1. What is CH4?
Methane (CH4) is a gas composed of carbon and hydrogen. Methane is the simplest of saturated hydrocarbons with a chemical formula CH4. It consists of four hydrogen atoms and one carbon atom and is the simplest alkane. It is colorless and odorless and cannot be detected by the human senses. It is flammable and is used to power vehicles, water heaters, and other appliances. CH4 is highly flammable at very high concentrations of about 50,000 ppm. Methane is considered an asphyxiant at extremely high concentrations. Methane is lighter than air, having a specific gravity of 0.554. It is only slightly soluble in water.
Methane is 80 times more potent than carbon dioxide at warming the climate system over the first 20 years after it’s released. Although methane breaks down more quickly than CO₂, its short-term impact on global warming is much greater, making it a critical target for environmental action.
Methane in Atmosphere:
Methane is the most abundant hydrocarbon in the atmosphere. It is produced by the decomposition of organic matter in the absence of oxygen by microorganisms (called methanogens). It is naturally present under the ground or underwater (seabed).
Methane is a short-lived climate pollutant. It readily converts to carbon dioxide (a less potent greenhouse gas), releasing other harmful air pollutants such as volatile organic compounds (VOCs) and ozone.
Methane, a powerful greenhouse gas, contributes to global warming by trapping heat inside the Earth’s atmosphere. It functions as a coolant in refrigerators and air conditioners. And in some industrial refrigerant applications, such as in the food industry. It is primarily used as natural gas.
In 2023, nearly 120 million tons of methane emissions were linked to fossil fuel activities. Around 80 million tons of that amount came from just the top 10 methane-emitting countries. The United States leads the world in methane emissions from oil and gas operations, with Russia coming in second. China, however, is the largest emitter of methane in the coal industry by a significant margin.
-
2. Sources
Methane (CH4) exists naturally and is produced by many sources. Methane gas can come from the following sources:
Methane enters the atmosphere through various sources, including landfills, agricultural activities, coal mining, stationary and mobile combustion, wastewater treatment, and specific industrial processes. According to the EPA, oil and gas production is one of the most significant sources of methane gas pollution.
- Methane is produced during the manufacture of some industrial compounds and used as fuel in various industrial activities.
- Methane is produced during wastewater treatment in sewage treatment facilities.
- Landfills are a major source of methane emissions, as organic waste degrades and generates methane gas.
- The digestive processes of animals produce CH4. Further, manure decomposition produces methane gas in the soil.
- Methane is a byproduct of coal mining and the extraction of oil and natural gas.
- Methane is produced when organic matter burns, such as in forest fires or agricultural burning.
Did you know this? Within agriculture, livestock farming, especially cattle farming, is the most significant source of methane emissions. In Germany, the agricultural sector accounts for around 75 percent of methane emissions.
Methane is the primary component of natural gas used for heating and cooking in your house. Natural gas is also used in several power plants to generate energy. Some methane is released when natural gas or crude oil is collected and delivered from oil and gas wells. One study found that about 13 million metric tons of methane leak into the atmosphere before it is even used. That is enough wasted gas to fuel 10 million homes for a year!
3. Permissible exposure limits for CH4
OSHA: The legal airborne permissible exposure limit (PEL) is 100 ppm, averaged over an 8-hour workshift.
NIOSH: The recommended airborne exposure limit is 100 ppm, averaged over a 10-hour work shift.
ACGIH: The recommended airborne exposure limit is 100 ppm, averaged over an 8-hour work shift.
Table 1. Methane exposure levels and effects.
Exposure level (ppm)
Effect or symptom
1000
NIOSH 8-hours TLV*
50,000 to 150,000
Potentially explosive
500,000
Asphyxiation
* TLV = Threshold Limit Value
Source – (https://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/agdex9038/$file/729-2.pdf?OpenElement&fbclid=IwAR1kqzolBzj46xk1fyTuQULkl3dsQiYdDI8LlIcnVLbVhEPQ33HIycI7v_c#:~:text=The%20Occupational%20Safety%20and%20Health,1%2C000%20ppm%20(0.1%20percent).
4. Health & Environmental Impact of CH4
Health Impact
Methane is a highly combustible gas that can cause major health problems if it accumulates in enclosed places or is consumed or inhaled. Some of the health risks connected with methane poisoning are:
- Respiratory irritation: Inhaling excessive methane levels can irritate the eyes, nose, and throat, resulting in coughing and wheezing.
- Explosive risk: Methane is highly flammable and easily ignited, posing an explosion risk in enclosed environments with large gas concentrations.
- Asphyxiation: Methane is an odorless and tasteless gas that can deplete oxygen in enclosed places, resulting in asphyxiation and death.
- Neurological consequences: When ingested at high doses, methane can produce headaches, dizziness, and nausea. Long-term exposure to low amounts of methane can harm the central nervous system.
Environmental Impact
Methane is a highly potent greenhouse gas, second to carbon dioxide, which makes it highly efficient in trapping heat. It is a major contributor to global warming.
CH4 is a major precursor of another greenhouse gas, carbon dioxide. While converting to CO2 in the atmosphere, methane reacts to form volatile organic compounds and leads to the formation of ground-level ozone when mixed with NOx.
5. Possible corrective measures
The primary action is CH4 monitoring, i.e., measuring how much CH4 concentrations you are exposed to. In addition to this, the following corrective measures can be taken:
- Regulations: Implementing the necessary rules on methane emissions can help to minimize the amount of gas released into the atmosphere.
- Training: Training personnel on how to spot and respond to methane exposure can help prevent poisonings.
- Maintenance: Regular maintenance of equipment and infrastructure helps prevent leaks and pollutants.
- Ventilation: Increasing airflow in regions with methane can dilute the concentration and lower the danger of poisoning.
- Avoid going to or staying in enclosed the source of CH4.
- If the presence of high levels of CH4 is detected, immediately vacate the area and provide proper ventilation to remove the gas.
- Avoid open disposal of animal waste.
- Also, promote the use of methane gas in applications such as cooking.
6. Measurement methods of CH4 monitoring
Different working principles for methane monitoring in the ambient environment are flame ionization detection (FID), semiconductor, electrochemistry, and nondispersive infrared absorption (NDIR).
Flame ionization detection (FID) – CH4 monitors based on the principle of FID uses a hydrogen flame to ionize the methane in the air. The ionized methane gas produces an electric current proportional to the concentration of methane in the sample air, which is detected by the detector.
Semiconductor – When a metal oxide semiconductor-based methane monitor is exposed to air samples, the CH4 molecules react on the metal oxide surface of the sensor and dissociate into charged ions, which alter the film’s resistance. This interaction is measured as a signal and is converted to the gas concentration.
Electrochemical – CH4 monitors working on the electrochemical principle are operated based on the diffusion of methane gas into the sensor, producing electrical signals proportional to the CH4 concentration.
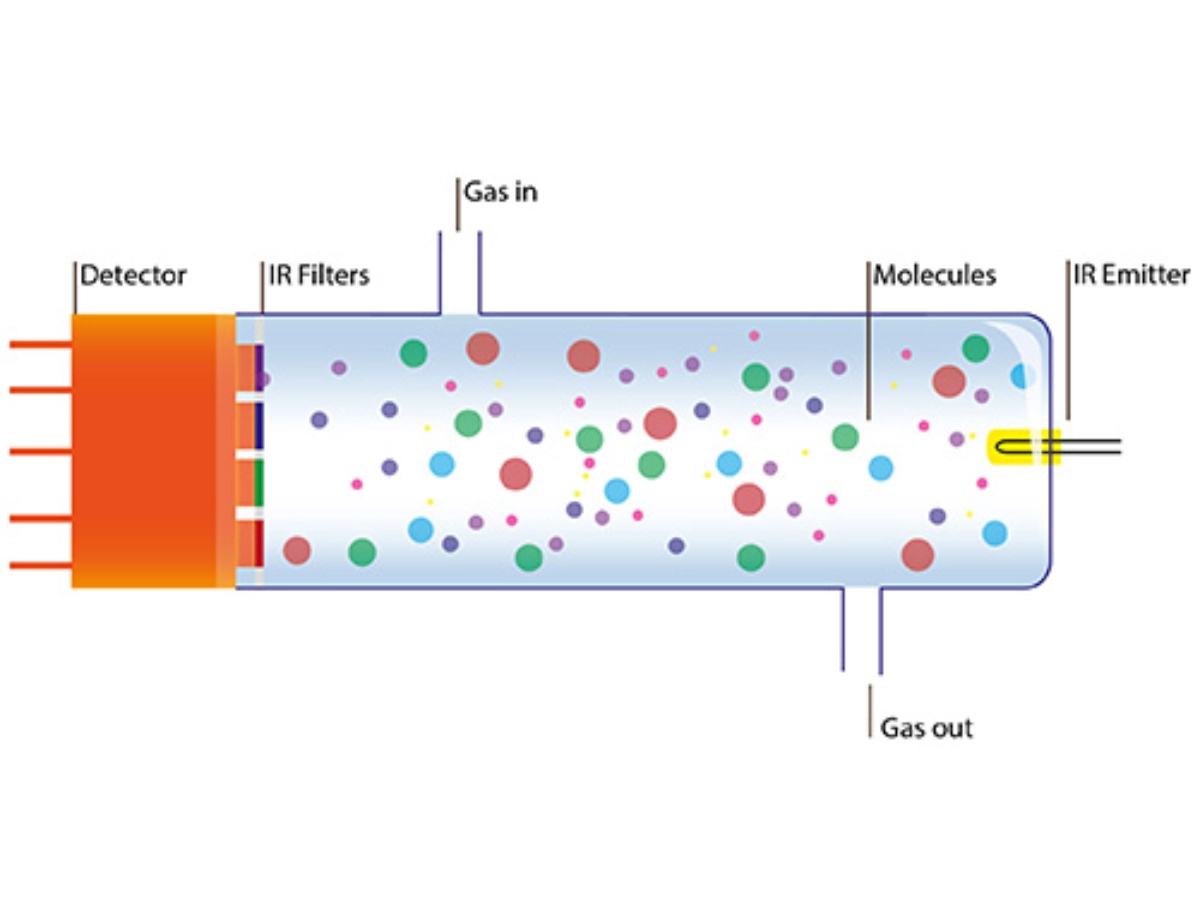
Nondispersive infrared absorption (NDIR) – Methane absorbs infrared radiation at a particular frequency. When the gas sample is exposed to infrared radiation, the infrared radiation absorbed by the CH4 molecules present in the gas sample is measured by the detector in a non-dispersive photometer.
[Source: https://www.lasercomponents.com/us/application/ndir-gas-analysis/ ]
7. Oizom’s sensor working principle for CH4 monitoring
Oizom provides a range of Chlorine (CH4) sensor modules to monitor varying CH4 levels based on your needs. Our sensors accurately measure CH4 in ambient conditions, detecting concentrations in ppb/ppm. This sensor monitors methane in real-time. This sensor is integrated into a metal casing and ultra-low-noise support electronics, making it compact and reliable. This allows accurate gas detection even at very low concentrations in the atmosphere. This sensor works on the Electrochemical working principle to measure environmental air quality.
This sensor undergoes calibration using standard gases and tools to ensure high sensitivity and accuracy. Each gas sensor is calibrated with zero and span checks in a controlled lab, following Section 12.2 of the USEPA Quality Assurance Handbook for Air Pollution Measurement Systems Volume II. Calibration is done using NIST-traceable gas standards for reliable performance.
This sensor module can be used in the AQBot single-parameter monitoring system and Odosense. It is designed to provide precise and reliable methane measurements, supporting various environmental monitoring and industrial processes. It is ideal for various applications, including landfill monitoring, mining monitoring, oil and gas industry operations, and research projects.
8. Why Choose Oizom CH4 Sensor?
- Compact: Our sensors are small and easy to install, perfect for use in any space, making them ideal for portable air quality monitoring. The CH4 sensors come pre-calibrated and can be quickly replaced in just a few minutes by removing and replacing the old sensor with a new one.
- Durable: The CH4 sensor has a long life of almost two years.
- Energy Efficient: There is no need to be concerned about energy usage because our sensors are not only accurate but also energy efficient. Powered up with just 3.3 to 5V for efficient, reliable performance!
- In-house sensor tech: Oizom applies advanced data processing algorithms to compensate for the effects of temperature and humidity on the sensor output. The algorithm is designed to automatically update based on weather conditions and seasonal changes, removing its influence on the sensor performance. The advanced algorithms also compensate for the effect of the cross-sensitive gas.
- Ultra-Low Noise Electronics: The sensor module’s design is crucial for accurate measurements. Each sensor is housed in a metal casing with ultra-low-noise electronics and a base PCB. The metal casing shields the sensor and electronics from electromagnetic interference, preventing false readings.
- RoHS Compliant: Our sensors comply with the RoHS criteria for restricting hazardous substances in electrical and electronic devices.
9. Reasons why CH4 monitoring is important
- Methane, a naturally occurring gas of carbon and hydrogen molecules, may be found in all four of Earth’s alchemical elements.
- The alchemists regarded water, earth, wind, and fire as the four classical elements making up our natural environment; methane has a place in each of these.
- CH4 is a colorless, odorless, tasteless, non-toxic gas that is naturally produced during the anaerobic decomposition of organic compounds. It tends to rise and accumulate near the higher, stagnant parts of enclosed spaces.
- On a 100-year timescale, methane has 28 times greater global warming potential than carbon dioxide and is 80 times more potent on a 20-year timescale.
- Its presence in the atmosphere increases the abundance of other greenhouse gases such as CO2, O3, water vapor, etc.
- CH4 is an asphyxiant at higher concentrations, leading to various issues such as suffocation, loss of consciousness, nausea, rapid breathing, numbness, etc., and may lead to coma and death.
- CH4 monitoring is an efficient way to detect the buildup of CH4 levels and take necessary actions.
- Real-time monitoring of CH4 levels helps determine their source and formulate an action plan to control CH4 emissions.
FAQs
- What is methane (CH4)?
Methane (CH4) is a colorless, odorless gas that is a potent greenhouse gas. It is mainly produced by natural sources and human activities like agriculture and fossil fuel extraction. - How does methane impact climate change?
Methane is over 28 times more effective than carbon dioxide at trapping heat in the atmosphere, making it a significant contributor to global warming. - Where does methane come from?
Methane is released from livestock digestion, landfills, natural gas leaks, and wetlands. - Is methane harmful to human health?
While methane is not toxic, it can reduce oxygen levels in enclosed spaces and contribute to ground-level ozone, harming respiratory health.
Reducing methane emissions can be achieved by improving waste management, reducing leaks in natural gas systems, and adopting sustainable agricultural practices.