HYDROGEN SULFIDE
Our environment is like a complex web where everything is connected. Some things, even though they seem small or unnoticeable, can greatly impact this system. Hydrogen Sulfide, or H2S for short, is one of those quiet but powerful players. Hydrogen sulfide (H₂S) is a colorless gas that smells like rotten eggs. While it arises naturally in locations such as swamps (forest wetlands) and hot springs, this gas is also a consequence of industrial processes, so it is important to understand it. H₂S is both a potential hazard and a desirable resource. It is dangerous in large doses, so sectors such as oil and gas monitor and limit its presence.
Given its dual nature of being both dangerous and beneficial, understanding hydrogen sulfide is crucial. Whether you’re involved in industries where it’s prevalent or simply curious about the gases around us, a deeper look into H₂S reveals its importance and the need for careful handling. So, as we explore hydrogen sulfide further, we’ll see that it’s more than just a foul smell; it’s a gas with significant implications in both industry and nature.
1. What is H2S?
H2S is a colorless, flammable, poisonous, and corrosive gas with one sulfur atom bonded to two hydrogen atoms. Hydrogen sulfide is structurally comparable to water. The Lewis structure of hydrogen sulfide consists of two hydrogen atoms and one sulfur atom. Sulfur is the central particle, consisting of two solitary hydrogens joined by a single bond.
It is also known as sewer, swamp, and manure. It has a noticeable rotten-egg odor detectable at concentrations as low as 0.5 ppb. It is a highly flammable, explosive gas between 4% and 45% (concentration in air), which may travel to ignition sources and flash back. When ignited, it burns to produce toxic vapors and gases (sulfur oxides). Hydrogen sulfide is slightly heavier than air. It may travel along the ground, collecting in low-lying, enclosed, or poorly ventilated areas such as basements, sewer lines, underground passages, manholes, etc.
Hydrogen sulfide (H2S) is a pungent chemical commonly associated with industrial sewage treatment and waste disposal. The US EPA does not list H2S as one of the six criteria health contaminants. However, legislation controlling the levels of H2S in ambient air is defined in most countries, and many jurisdictions have ambient exposure standards for H2S.
Hydrogen sulfide in Atmosphere:
H2S is an important player in the global sulfur cycle. It is oxidized in the environment to SO2, which is subsequently transformed to sulfate via three distinct chemical processes. H2S is slightly soluble in water, causing the development of sulfhydric acid, which is corrosive to metals and leads to acidic deposition in soil and water.
Hydrogen sulfide is typically a byproduct of sulfur-containing coal production, oil refining, and wood pulp manufacture. However, it is a crucial reagent or intermediate in certain processes, including producing sulphuric acid, inorganic sulfides, and agricultural disinfectants, as from any such source where it is produced from the bacterial breakdown (anaerobic decay) of organic sulfur compounds. Hydrogen sulfide remains in the atmosphere for approximately 1–42 days, depending on the season. It can change into sulfur dioxide and sulfates in the air.
Once released, it remains in the atmosphere for about 18 hours before converting to other sulfur oxides. H2S in the air oxidizes to form SO2, which consequently reacts to form sulfate particles. It also contributes to acid rain by dissolving into water vapor to form sulfuric acid.
Did you know this? In an instance, hydrogen sulfide generated by an industrial waste pool (around 300 ppb ambient levels) caused, apart from the bad smell, nausea, vomiting, headaches, loss of appetite, and sleep disturbances to nearby residents (WHO, 1981).
2. Sources
Hydrogen sulfide can also be released from industrial sources such as petroleum refineries, natural gas plants, kraft paper mills, manure treatment facilities, wastewater treatment facilities, and tanneries.
- Crude Petroleum: Crude petroleum may emit H2S gas during extraction and processing, posing health and safety risks.
- Sewage Treatment: Sewage treatment plants may release H2S gas as the organic component in wastewater breakdowns.
- Refineries: Refineries can produce H2S gas by refining sulfur-containing crude oil. The gas might be harmful to one’s health and safety.
- Kraft Paper Mills: Kraft paper mills use sulfur-based compounds in the pulping process, producing H2S gas.
- Food Processing: Food processing facilities that use sulfur and sulphuric acid generate H2S gas in the surrounding environment.
- Wastewater Treatment: Wastewater treatment plants produce H2S gas during the breakdown of organic matter.
3. Permissible exposure limits for H2S
|
8-hr. TWA |
STEL |
Ceiling Limit |
Acceptable Max Peak Above Ceiling for an 8-Hour Shift |
|
|---|---|---|---|---|
|
Federal OSHA PEL |
NA |
NA |
20 ppm |
50 ppm (maximum duration-10 minutes |
|
NIOSH REL |
10 ppm |
15 ppm |
NA |
NA |
|
ACGIH TLV (2010) |
1.0 ppm |
5.0 ppm |
NA |
NA |
Workplace Exposure Limit:
- OSHA: The legal airborne permissible exposure limit (PEL) is 20 ppm, not to be exceeded at any time, and 50 ppm, as a maximum peak, not to be exceeded during any 10-minute work period.
- NIOSH: The recommended airborne exposure limit (REL) is 10 ppm, which should not be exceeded during any 10-minute work period.
- ACGIH: The threshold limit value (TLV) is 1 ppm averaged over an 8-hour work shift and five ppm as an STEL (short-term exposure limit).
The exposure limits mentioned apply only to air. If your skin also comes into contact, you could still be overexposed, even if the air levels are below the listed limits.
Permissible Exposure Limit (PEL) given by OSHA (Occupational Safety and Health Administration) defines the maximum concentration of H2S to which an unprotected worker may be exposed. PEL may reference an eight-hour time-weighted average (TWA), a 15-minute short-term exposure limit (STEL), or an instantaneous ceiling limit (CL) concentration that cannot be exceeded for any period. Similarly, the Recommended Exposure Limit (REL) is the occupational exposure limit recommended by NIOSH (National Institute for Occupational Safety and Health), and the TLVs (i.e., threshold limit values) are the exposure guidelines given by ACGIH (American Conference of Governmental Industrial Hygienists).
4. Health & Environmental Impact of H2S
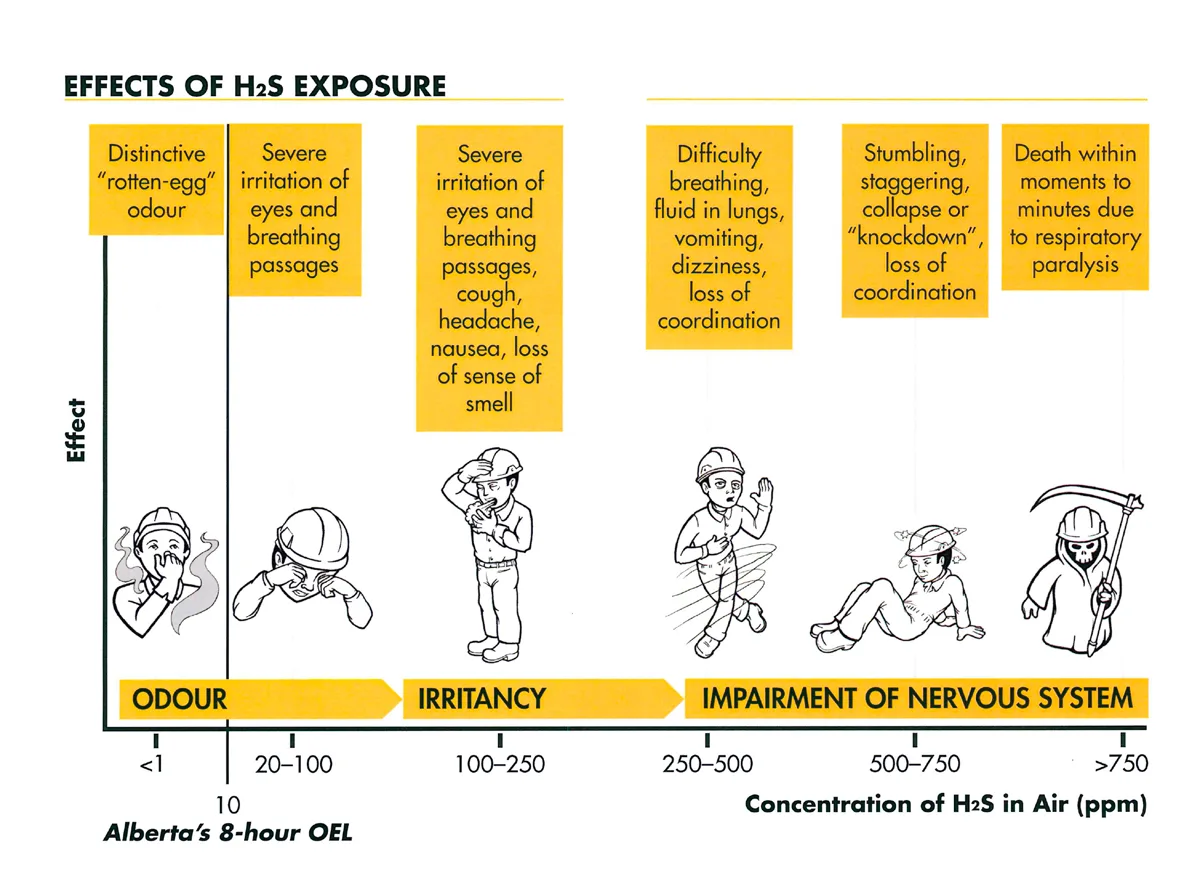
H2S is a highly flammable gas that can react with steel at ambient temperature, which makes handling, storage, transportation, and working with it extremely laborious and demanding for workers, laborers, miners, and others who are required to work with it on a regular basis. Hydrogen sulfide (H2S) primarily affects the respiratory system, with initial symptoms including nose, throat, and eye irritation.
Human smell detects this chemical at far lower amounts than those that are harmful to health. Short-term exposure to high amounts might result in headaches, dizziness, and vomiting. However, with continued exposure and at high concentrations, it causes olfactory fatigue i.e., the person loses the ability to smell H2S even if it is present. At very high concentrations, the ability to smell the gas can be lost instantaneously. Its health effects can vary depending on the level and duration of exposure.
H2S impairs cellular respiration by inhibiting cytochrome c oxidase, an essential enzyme for oxygen utilization. This can result in:
- Cеllular hypoxia is a lack of oxygen at the cellular level.
- Mitochondrial failure causes a decrease in energy generation in cells.
- Acidеmia: Acidification of the blood caused by H2S’s low acidic properties.
[https://hnhu.org/health-topic/what-is-hydrogen-sulphide-h2s/]
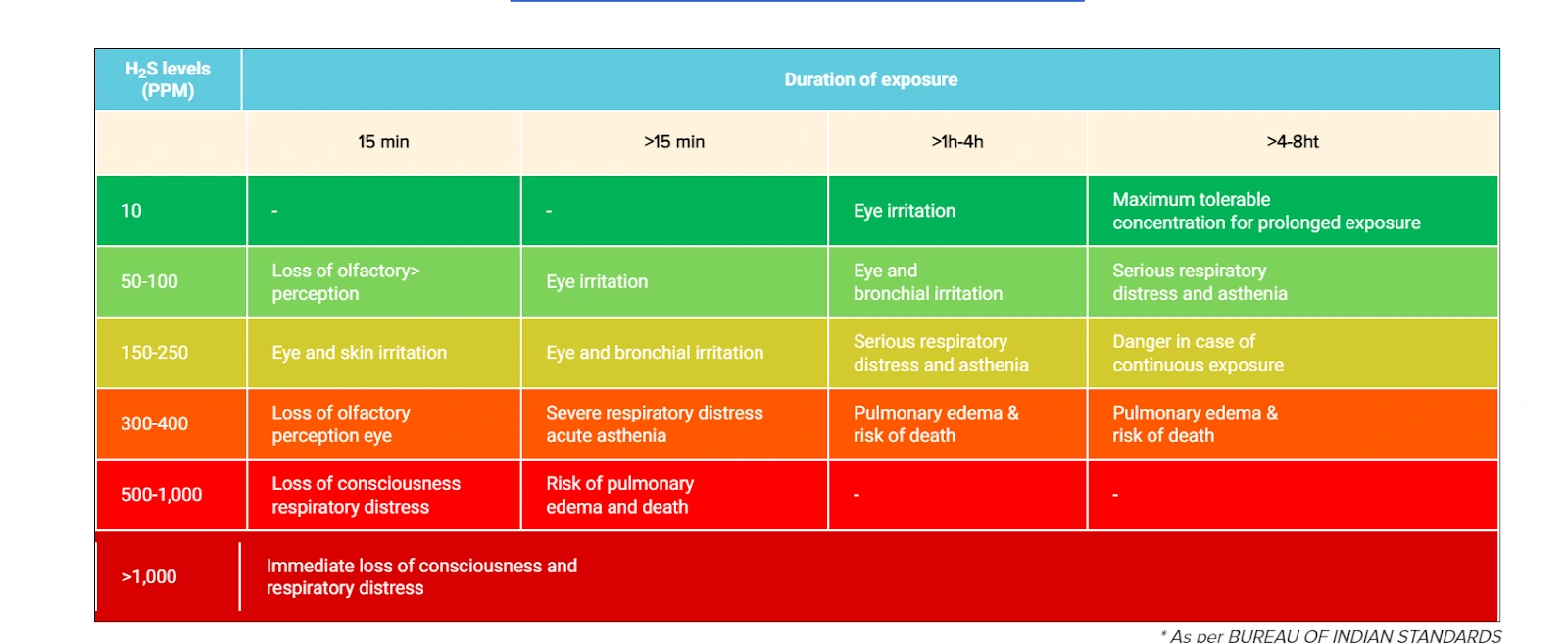
Keeping in mind the acute health risks of occupational hydrogen sulfide emissions, the table below displays the Industrial Safety and Chemical Hazards Sectional Committee’s H2S values in PPM.
Environmental Impact
Hydrogen sulfide degrades in the atmosphere and disappears without an ongoing source. It is transformed into sulfur dioxide and then sulfates, which are taken by plants and soil or eliminated by precipitation like rain or snow. This process takes 1-2 days in warm weather and up to several weeks in cold weather, as cooler temperatures allow hydrogen sulfide and its breakdown products to persist in the outdoor air for longer. Additionally, it is capable of corroding metals.
H2S oxidizes in the atmosphere to sulfur dioxide (SO2), a critical ambient air pollutant, which negatively affects human health and the environment. It further reacts to form sulfates through several different pathways. Though not directly involved in climate change.
5. Possible corrective measures
The primary action is H2S monitoring, i.e., measuring how much H2S concentrations you are exposed to. In addition to this, the following corrective measures can be taken:
- Improve Ventilation: Ensure proper airflow in areas where H2S might accumulate to reduce concentration and improve air quality. Hydrogen sulfide is heavier than air. It can easily travel to any source of ignition.
- Install Gas Detectors: Use H2S sensors to monitor levels in real-time and alert you to dangerous concentrations.
- Use Scrubbers or Filters: Implement scrubbers or filtration systems to remove H2S from air or gas streams.
- Limit Exposure: Restrict access to areas with high H2S levels and enforce personal protective equipment (PPE) use.
- Regular Maintenance: Inspect and maintain equipment regularly to prevent leaks or buildup of H2S.
6. Measurement methods of H2S monitoring
Hydrogen sulfide in the environment can be monitored using several common methods. These include pulsed UV fluorescence, the lead acetate tape method, semiconductor sensors, and electrochemical techniques.
Pulsed U.V. Fluorescence – The H2S monitor working on the UV fluorescence principle is based on the emission of a characteristic fluorescence when SO2 molecules are irradiated by UV light. The air sample taken by the H2S monitor takes two routes. One part of the sample passes through the converter, where the H2S gas is converted to SO2 by a catalytic reaction, while the other part bypasses the converter. When SO2 is exposed to the beam of UV radiation in the region of 190-230 nm, it gets excited and emits a characteristic fluorescence radiation of 320-380 nm when it returns to its ground state. The radiation is passed through a filter and is recorded by the detector (photomultiplier tube). The difference between the signal from both parts is inferred as the concentration of H2S. Potential interferences in hydrogen sulfide monitors include water vapor, nitric oxide (NO), and various hydrocarbons.
Lead Acetate Tape Method – The H2S monitor based on this method relies on the chemical reaction of H2S with lead acetate-impregnated paper tape to form lead sulfide. The color of the lead acetate tape changes if the H2S is present in the sensor, as lead sulfide appears as a brown stain on the paper tape. A light source is used to illuminate the tape, and specially calibrated optics measure the H2S concentration based on the slight variations in the depth of the tape’s color change.
Semiconductor – When a metal oxide semiconductor-based hydrogen sulfide monitor is exposed to an air sample, the H2S molecules react on the metal oxide surface of the sensor and dissociate into charged ions that alter the film’s resistance. This interaction is measured as a signal and is converted to the gas concentration. However, the energy consumption of such H2S monitors is higher compared to others.
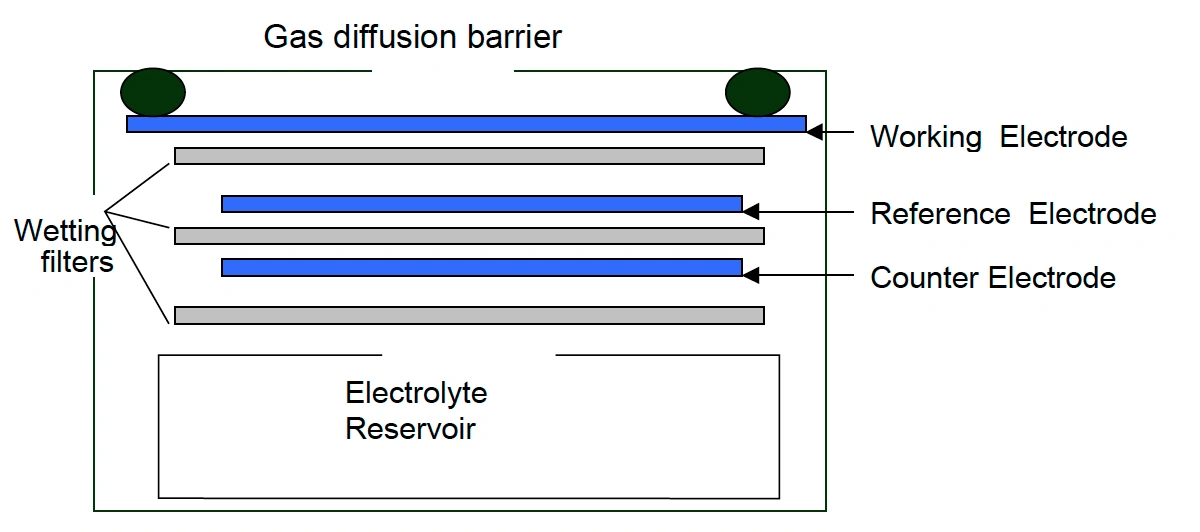
Electrochemical – H2S monitors working on the electrochemical principle are operated based on the diffusion of hydrogen sulfide gas into the sensor, producing electrical signals proportional to the H2S concentration. It allows accurate measurement of even low concentrations of H2S, which is essential in H2S monitoring in the ambient air.
[Source: https://docs.smartcitizen.me/Components/sensors/Electrochemical%20Sensors/]
7. Oizom’s sensor working principle for H2S monitoring
Oizom provides a range of Hydrogen sulfide (H2S) sensor modules to monitor varying H2S levels based on your needs. Our sensors accurately measure H2S in ambient conditions, detecting concentrations in ppb/ppm. This sensor monitors Hydrogen sulfide in real time. This sensor is integrated into a metal casing and ultra-low-noise support electronics, making it compact and reliable. This allows accurate gas detection even at very low concentrations in the atmosphere. This sensor works on the Electrochemical working principle to measure environmental air quality.
This sensor undergoes calibration using standard gases and tools to ensure high sensitivity and accuracy. Each gas sensor is calibrated with zero and span checks in a controlled lab, following Section 12.2 of the USEPA Quality Assurance Handbook for Air Pollution Measurement Systems Volume II. Calibration is done using NIST-traceable gas standards for reliable performance.
The H2S sensor module is integrated into outdoor air quality monitoring systems like AQBot and Odosense. It is ideal for sewage, effluent treatment, industries, landfills, solid waste management, and air quality research projects. By utilizing this sensor module, users can ensure they receive accurate, real-time data on H2S levels, aiding in effectively monitoring and managing air quality across diverse environments.
8. Why Choose Oizom H2S Sensor?
- Compact: Our sensors are small and easy to install, perfect for use in any space, making them ideal for portable air quality monitoring. The H2S sensors come pre-calibrated and can be quickly replaced in just a few minutes by removing and replacing the old sensor with a new one.
- Durable: The H2S sensor has a long life of almost two years.
- Energy Efficient: There is no need to be concerned about energy usage because our sensors are not only accurate but also energy efficient. Powered up with just 3.3 to 5V for efficient, reliable performance!
- In-house sensor tech: Oizom applies advanced data processing algorithms to compensate for the effects of temperature and humidity on the sensor output. The algorithm is designed to automatically update based on weather conditions and seasonal changes, removing its influence on the sensor performance. The advanced algorithms also compensate for the effect of the cross-sensitive gas.
- Ultra-Low Noise Electronics: The sensor module’s design is crucial for accurate measurements. Each sensor is housed in a metal casing with ultra-low-noise electronics and a base PCB. The metal casing shields the sensor and electronics from electromagnetic interference, preventing false readings.
- RoHS Compliant: Our sensors comply with the RoHS criteria for restricting hazardous substances in electrical and electronic devices.
9. Reasons why H2S monitoring is important
- H2S is a colorless, odorous gas that is majorly produced naturally during the anaerobic decay of organic sulfur compounds and can accumulate in enclosed, poorly ventilated, and low-lying areas.
- H2S, apart from affecting human health and the environment itself, produces other pollutants, such as sulfur dioxide and sulfuric acid, which have negative effects on health and the environment.
- When inhaled, H2S rapidly gets absorbed by the lungs, leading to respiratory problems, paralysis, and nervous system impairment. It is important to monitor the levels of H2S we are exposed to.
- It is very unlikely that the general population will be exposed to a level of hydrogen sulfide high enough to cause adverse health effects.
- An air quality monitor/sensor detects H2S gas and provides real-time information on its atmospheric concentration. This information can help make informed decisions to protect public health and the environment.
FAQs
What is hydrogen sulfide?
Hydrogen sulfide is a colorless, flammable gas with a characteristic odor of rotten eggs.
How will I be exposed to hydrogen sulfide?
You may be exposed to hydrogen sulfide or skin and eye contact by breathing it in.
What happens when hydrogen sulfide reacts with oxygen?
The reaction depends on the amount of available oxygen:
- Sufficient Oxygen: 2H₂S + 3O₂ → 2SO₂ + 2H₂O. This complete combustion generates sulfur dioxide (SO₂) and water.
- Limited Oxygen: 2H₂S + O₂ → 2S + 2H₂O. Incomplete combustion produces elemental sulfur instead of SO₂. This reaction often occurs in low-oxygen environments, like volcanic springs or sewers.
What happens if you breathe in H2S?
The severity of the effects is determined by the concеntration and exposure time:
- Low Lеvеls (1-10 ppm): Mild odor, eye irritation, and respiratory discomfort.
- Moderate levels (10-250 ppm): Coughing, nausеa, dizzinеss, and hеadachеs at Prolonged exposure can result in pulmonary еdеmа (fluid buildup in the lungs).
- High levels (250 ppm or higher) cause rapid unconsciousness, respiratory paralysis, and death.
What does hydrogen sulfide do to the body?
H2S can irritate the eyes, nose, and throat, cause headaches, dizziness, and breathing issues, and, in high concentrations, lead to serious health risks like unconsciousness or respiratory failure.
What is the PEL of hydrogen sulfide?
The Occupational Safety and Health Administration’s (OSHA) Permissible Exposure Limit (PEL) for hydrogen sulfide is:
- The upper limit is 20 parts per million (ppm).
- For 10 minutes, the most significant peak was 50 ppm.
These limitations prevent harmful effects and maintain worker safety in H2S-contaminated workplaces. Regular monitoring and adherence to these limits is critical in industrial and other high-risk environments.