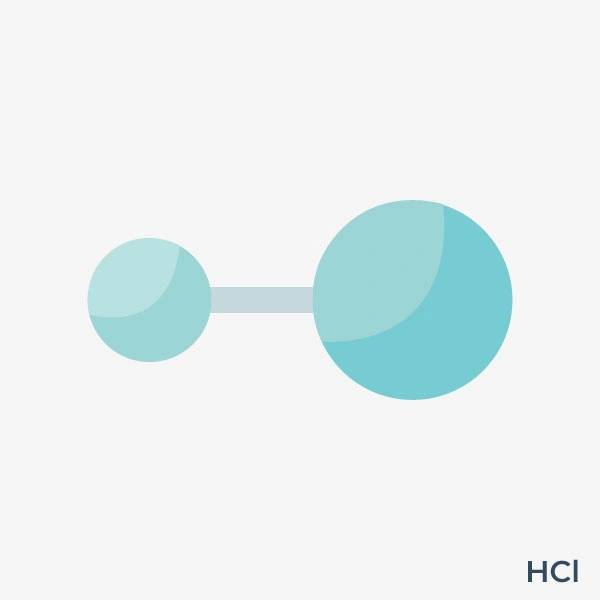
Hydrogen chloride (HCl) is a highly reactive, colorless gas with a sharp, pungent odour. Composed of hydrogen and chlorine, hydrogen chloride was first made in 1648 by Glauber by heating sodium chloride with concentrated sulfuric acid. It is widely used across industries due to its versatile chemical properties. When dissolved in water, it forms hydrochloric acid. HCl plays a significant role in industries like chemical manufacturing, pharmaceuticals, and metallurgy. It is a key ingredient used to make chlorine-based compounds, PVC plastics, and various organic chemicals.
For environmental professionals, understanding HCl is essential for addressing environmental challenges. Monitoring HCl levels is crucial for assessing the impact of mitigation strategies. This article provides detailed insights into hydrogen chloride (HCl), including its sources in ambient air, concentration levels, impacts on health and the environment, corrective actions, the importance of HCl monitoring, and the various technical methods used for HCl detection and measurement.
1. What is HCl?
Hydrochloric acid is a strong, corrosive inorganic acid with the chemical formula HCl. It is also called hydrogen chloride or muriatic acid. Hydrogen chloride, with the chemical formula HCl, is a type of hydrogen halide. At room temperature, it is a colorless gas that creates white fumes of hydrochloric acid when it comes into contact with water vapor in the air. Both the gas and the acid are widely used in various industries and technologies.
Hydrogen chloride is a colorless to slightly yellow gas with a sharp, irritating smell. While it is not flammable, it can react with substances like alcohol, hydrogen cyanide, or aluminum-titanium alloys to form combustible compounds. When dissolved in water, it forms a strong, highly corrosive acid. This makes HCl a strong irritant to the eyes, nose, and throat. Even brief exposure to levels as low as 35 ppm can cause throat irritation.
When hydrogen chloride dissolves in water, it forms HCl. It is a simple molecule with one hydrogen atom and one chlorine atom connected by a single covalent bond. The bond is polar because chlorine is more electronegative than hydrogen. It is commonly made in both laboratories and industries by reacting a chloride, usually sodium chloride (NaCl), with sulfuric acid (H₂SO₄). It can also be produced by reacting certain chlorides, like phosphorus trichloride (PCl₃) or thionyl chloride (SOCl₂), with water. Additionally, it is a by-product of chlorinating organic compounds, such as methane or benzene. It is a poisonous, corrosive, hazardous liquid that reacts with most metals to form explosive hydrogen gas and causes severe burns and irritation of the eyes and mucous membranes.
Hydrogen Chloride in Atmosphere:
Hydrogen chloride (HCl) is released into the atmosphere through natural events like volcanic eruptions and human activities such as industrial processes. In the air, it reacts with water vapor to form hydrochloric acid, which contributes to acid rain. Acid rain can harm plants, aquatic life, and buildings by making the environment more acidic.
HCl in the atmosphere can also affect air quality, especially in areas near industries or volcanoes. Breathing in even small amounts of hydrogen chloride can irritate the eyes, throat, and lungs, while higher levels can be dangerous.
Exposure to hydrogen chloride in the atmosphere can irritate the respiratory system, eyes, and skin. Even low levels can cause discomfort, while higher concentrations pose serious health risks. This makes it crucial to monitor and manage HCl emissions effectively. By understanding its effects, we can work toward minimizing its harm and promoting a cleaner environment.
2. Sources
Hydrogen chloride (HCl) is released into the environment through both natural and human activities.
- Natural Sources: Volcanic eruptions release significant amounts of hydrogen chloride into the atmosphere.
- Industrial Production: Produced during the manufacturing of chemicals and plastics, such as PVC, and emitted during metal treatment and refining processes.
- Hydrochloric acid is widely used in various industries. It is essential for producing chlorides, refining tin, and tantalum ore, and pickling or cleaning metal products. It is also used in electroplating, removing scale from boilers, neutralizing basic systems, and as a reagent in laboratories. Additionally, it acts as a catalyst and solvent in organic synthesis, helps manufacture fertilizers and dyes, and is used to hydrolyze starch and proteins for food preparation. Other applications include the photographic, textile, and rubber industries.
- Due to its widespread use, hydrogen chloride is released into the air from anthropogenic activities such as waste incinerators or chemical plants. It is formed in large quantities when materials such as plastics or polyvinyl chloride (PVC) are burnt.
By-product Formation: Generated as a by-product in industrial reactions, such as the chlorination of organic compounds like methane or benzene.
Laboratory Processes: Formed by reacting chlorides (e.g., sodium chloride) with sulfuric acid.
3. Levels of HCl
Hydrogen chloride (HCl) is highly soluble, meaning exposure often involves both gas and aerosol. It can severely irritate the eyes and mucous membranes, and contact with the skin may cause burns or inflammation. Prolonged exposure to HCl mist can lead to tooth erosion and ulcers inside the nose. Inhaling HCl can cause coughing, choking, and swelling in the respiratory system, depending on the exposure level. Health effects at various concentration levels are summarized in the table.
Health effects of respiratory exposure to hydrogen chloride
| Exposure Limits (PPM) | Health Effects |
| 5< | Coughing |
| 35 | Throat irritation occurs after only a short time |
| 35< | Severe breathing difficulties and skin inflammation or burns |
| 10-50 | Maximum level that can be sustained for several hours |
| 100< | Swelling of the lungs and often throat spasms |
| 50-1000 | Maximum possible exposure = 1 hour |
| 1000-2000 | Very dangerous, even for a very short exposure |
(Source – https://www.ivhhn.org/information/information-different-volcanic-gases/hydrogen-chloride )
Existing Guidelines: Only occupational guidelines exist for gaseous HCl, and these are given in the table.
Occupational Guidelines for HCl
| Country/Institution | Level (PPM) | Level µg m-3 | Averaging Period |
| EU | 5 | 8000 | 8 Hour TWA |
| EU | 10 | 15000 | STEL |
| UK | 5 | 8000 | 15 min |
| UK | 1 | 2000 | 8 hour TWA |
| USA | 5 | 7000 | 8 hour TWA |
| USA | 5 | 2000 | ceiling |
| USA | 3 | 1 hour | |
| USA | 20 | 1 hour |
(Source – https://www.ivhhn.org/information/information-different-volcanic-gases/hydrogen-chloride )
Workplace Exposure Limit:
- OSHA: The legal airborne permissible exposure limit (PEL) is 5 ppm, not to be exceeded at any time.
- NIOSH: The recommended airborne exposure limit (REL) is 5 ppm, which should not be exceeded at any time.
- ACGIH: The threshold limit value (TLV) is 2 ppm, which should not be exceeded at any time.
(Source – https://nj.gov/health/eoh/rtkweb/documents/fs/1012.pdf)
4. Health & Environmental Impact of HCl
Hydrogen chloride (HCl) poses significant health and environmental risks when not handled properly.
- Hydrochloric acid is highly corrosive and can cause severe irritation to the eyes, skin, and mucous membranes. Inhalation may lead to coughing, hoarseness, chest pain, inflammation, and even ulceration of the respiratory tract.
- Severe cases can result in pulmonary edema. Skin contact may lead to severe burns, ulceration, and permanent scarring.
- While inhaling, its fumes can lead to coughing, choking, and swelling in the throat and lungs. Prolonged exposure to HCl mist may erode tooth enamel and cause nasal ulcers, making workplace safety crucial for industries handling it.
Did you know this? The International Agency for Research on Cancer (IARC) has designated hydrochloric acid as being not classifiable as to its carcinogenicity to humans (category 3: there was inadequate evidence in humans or experimental animals)
Environmental Impact
Hydrogen chloride (HCl) can have significant environmental impacts, especially in ambient air, if not managed responsibly. In the atmosphere, HCl reacts with water vapor to form hydrochloric acid, contributing to acid rain. Acid rain lowers the pH of soil and water bodies, harming plants, aquatic life, and microorganisms. This disrupts ecosystems, damages crops, and corrodes buildings and infrastructure.
In ambient air, HCl exists as a gas or aerosol and poses a direct threat to air quality. It can react with other pollutants, forming secondary pollutants that exacerbate environmental degradation. Prolonged exposure in industrial areas with high HCl emissions can lead to respiratory issues for nearby populations and affect local vegetation. The dispersion of HCl in ambient air is particularly harmful in urban areas, where it combines with moisture to create localized acidic conditions.
5. Possible Corrective Measures
The primary action is HCl monitoring, i.e., measuring how much HCl concentrations you are exposed to. In addition to this, the following corrective measures can be taken:
- Emission Control: Industries should use advanced filtration systems, like scrubbers, to capture HCl emissions before they enter the atmosphere. These systems can significantly reduce the release of harmful gases, ensuring cleaner air.
- Proper Storage and Handling: HCl should be stored in corrosion-resistant containers and handled with care to prevent leaks or spills. Ensuring proper ventilation in storage and work areas reduces the risk of accidental exposure.
- Neutralization Measures: Spraying alkaline solutions like sodium bicarbonate near emission sources can help neutralize airborne HCl before it spreads widely, reducing its impact on ambient air quality.
- Ambient Air Monitoring: Regular monitoring of air quality in industrial areas helps detect HCl levels early. Using real-time air quality monitoring devices ensures timely action to prevent harmful exposure to workers and nearby communities.
- Regulatory Compliance and Training: Adhering to environmental standards and providing proper training to workers on HCl handling and emergency measures can prevent accidents and long-term impacts.
6. Measurement Methods of HCl Monitoring
Hydrogen chloride in the environment can be monitored using several common methods. These include electrochemical, Photoacoustic Spectroscopy (PAS), and Fourier-Transform Infrared Spectroscopy (FTIR).
Photo-acoustic: Photoacoustic sensors for hydrogen chloride (HCl) operate on the principle of light absorption and sound detection. Unlike NDIR sensors, instead of measuring the light received by a detector like NDIR sensors, photo-acoustic sensors use a microphone to detect sound. When HCl molecules absorb infrared (IR) light, this absorption causes the molecules to vibrate and produce a faint “humming” sound, or pressure waves, which are detected by a sensitive microphone placed near the sample. The key advantage of this method is that it doesn’t require a clear line of sight, allowing these sensors to be much smaller in size; some are even less than 1 cm³.
Fourier-Transform Infrared Spectroscopy (FTIR): it is a reliable technique for detecting and quantifying hydrogen chloride (HCl) by analyzing its unique infrared (IR) absorption properties. The principle is based on the fact that HCl molecules absorb specific wavelengths of IR light, which correspond to their molecular vibrations.
In an FTIR system, an IR light source emits a broad spectrum of light, which is directed through a gas sample containing HCl. As the light interacts with the sample, the HCl molecules absorb specific wavelengths that match their natural vibrational frequencies. The remaining unabsorbed light continues through the system and is collected for further analysis.
The transmitted light is passed through an interferometer, which modulates it into an interference pattern. This raw data is then processed using a mathematical technique called Fourier Transform, which converts the pattern into an absorption spectrum. The spectrum serves as a molecular “fingerprint,” identifying the presence of HCl and allowing for precise concentration measurement.
Electrochemical: Electrochemical sensors detect hydrogen chloride (HCl) by measuring the electrical changes caused by its chemical reaction within the sensor. These sensors are compact, reliable, and widely used for real-time monitoring in industrial and environmental applications.
The sensor consists of three main components: a sensing electrode, a counter electrode, and an electrolyte. When HCl gas enters the sensor, it passes through a gas-permeable membrane and interacts with the electrolyte. This interaction triggers a chemical reaction, typically involving the oxidation or reduction of HCl molecules.
As the reaction occurs, it generates an electrical current proportional to the concentration of HCl in the air. The sensor’s internal electronics measure this current and convert it into a readable output that indicates the HCl concentration. Electrochemical sensors are highly selective, responding specifically to HCl and minimizing interference from other gases.
7. Oizom’s Sensor Working Principle for HCl Monitoring
Oizom provides a range of Hydrogen chloride (HCl) sensor modules to monitor varying HCl levels based on your needs. Our sensors accurately measure HCl in ambient conditions, detecting concentrations in ppb/ppm. This sensor monitors Hydrogen chloride in real-time. This sensor is integrated into a metal casing and ultra-low-noise support electronics, making it compact and reliable. This allows accurate gas detection even at very low concentrations in the atmosphere. This sensor works on the Electrochemical working principle to measure environmental air quality.
This sensor undergoes calibration using standard gases and tools to ensure high sensitivity and accuracy. Each gas sensor is calibrated with zero and span checks in a controlled lab, following Section 12.2 of the USEPA Quality Assurance Handbook for Air Pollution Measurement Systems Volume II. Calibration is done using NIST-traceable gas standards for reliable performance.
The HCl sensor module is integrated into outdoor air quality monitoring systems like AQBot, Pollusense, and Odosense. It is ideal for monitoring HCl at the manufacturing of chemical plants, plastics, and waste incinerators. By utilizing this sensor module, users can ensure they receive accurate, real-time data on HCl levels, aiding in effectively monitoring and managing air quality across diverse environments.
8. Why Choose Oizom HCl Sensor?
- Compact: Our sensors are small and easy to install, perfect for use in any space, making them ideal for portable air quality monitoring. The HCl sensors come pre-calibrated and can be quickly replaced in just a few minutes by removing and replacing the old sensor with a new one.
- Durable: The HCl sensor has a long life of almost two years.
- Energy Efficient: There is no need to be concerned about energy usage because our sensors are not only accurate but also energy efficient. Powered up with just 3.3 to 5V for efficient, reliable performance!
- In-house sensor tech: Oizom applies advanced data processing algorithms to compensate for the effects of temperature and humidity on the sensor output. The algorithm is designed to automatically update based on weather conditions and seasonal changes, removing its influence on the sensor performance. The advanced algorithms also compensate for the effect of the cross-sensitive gas.
- Ultra-Low Noise Electronics: The sensor module’s design is crucial for accurate measurements. Each sensor is housed in a metal casing with ultra-low-noise electronics and a base PCB. The metal casing shields the sensor and electronics from electromagnetic interference, preventing false readings.
- RoHS Compliant: Our sensors comply with the RoHS criteria for restricting hazardous substances in electrical and electronic devices.
9. Reasons Why HCl Monitoring is Important:
- Hydrogen chloride (HCl) is a highly reactive, colorless gas with a sharp, pungent odour. While it is not flammable, it can react with substances like alcohol, hydrogen cyanide, or aluminum-titanium alloys to form combustible compounds.
- HCl is a highly corrosive gas with strong acidic properties. It can easily irritate the eyes, throat, and respiratory system, even at low concentrations. Prolonged exposure may lead to lung damage, nasal ulcers, or severe respiratory distress.
- Hydrogen chloride (HCl) is released into the atmosphere through natural events like volcanic eruptions and human activities such as industrial processes. In the air, it reacts with water vapor to form hydrochloric acid, which contributes to acid rain.
- HCl contributes to acid rain when it reacts with moisture, damaging crops, aquatic ecosystems, and infrastructure.
- Given the serious consequences for human health and the environment, monitoring HCl is critical. By monitoring odourful gas levels, we can determine when they are dangerously high.
- Real-time hydrogen chloride monitoring helps calculate the concentration in the air to deliver health advisories and formulate an action plan to meet standards.
FAQs
1. What is hydrogen chloride (HCl), and where is it found
Hydrogen chloride (HCl) is a highly reactive, colorless gas with a sharp odour. It is found in industrial emissions and volcanic gases and is used in manufacturing processes like PVC production.
2. How does hydrogen chloride impact air quality?
HCl reacts with water vapor in the air to form hydrochloric acid, contributing to acid rain. It can also degrade air quality near industrial areas, posing risks to health and the environment.
3. Why is monitoring hydrogen chloride important?
Monitoring HCl helps prevent health issues like respiratory irritation and environmental damage like acid rain. Real-time data ensures compliance with safety standards and effective mitigation.
4. What industries release hydrogen chloride into the atmosphere?
Industries like chemical manufacturing, plastics production, and waste incineration emit HCl during processes such as chlorination or combustion of materials like PVC.
5. What are the best methods to monitor hydrogen chloride?
Hydrogen chloride is monitored using electrochemical sensors, FTIR spectroscopy, or photoacoustic sensors, which provide accurate real-time data on ambient air concentrations.