Methyl mercaptan (CH3SH), often known as methanethiol, is a poisonous, highly flammable, colorless gas that smells like rotten cabbage. It can be found naturally in the blood, brain, and various animal and plant tissues. It is one of the key compounds responsible for bad breath and the unpleasant odour of flatulence. Its presence in the environment can be a cause for concern.
Prolonged exposure of the CH3SH can quickly deaden a person’s sense of smell, making the odour of methyl mercaptan an unreliable indicator of its presence. Hence, other means such as use of methyl mercaptan monitors is a viable solution to provide adequate warning of hazardous exposure. This article covers information on methyl mercaptan, its sources in the ambient air, permissible levels, health and environmental impact, possible corrective measures, need for methyl mercaptan monitors as well as different methods of CH3SH monitoring.
What is Methyl Mercaptan?
Methyl mercaptan (CH3SH), also known as methanethiol, is a colorless, highly flammable, toxic gas with a distinct, strong unpleasant odour. Its odour can be described as that of rotten cabbage or garlic. According to Central Pollution Control Board’s (CPCB) report, the odour of methyl mercaptan can be detected at a concentration of 0.0005 ppm. Most humans can detect methyl mercaptan odour at concentrations 500 times lower than the accepted safe exposure limit.
Table: Odour Intensity of Methyl Mercaptan
|
Intensity |
Description |
Concentration (ppm) |
|
0 |
No odour |
0.0030 |
|
1 |
Threshold |
0.041 |
|
2 |
Faint |
0.57 |
|
3 |
Median, easily noticeable |
7.9 |
|
4 |
Strong |
110 |
|
5 |
Most Intense |
1500 |
[Source: Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 15, National Research Council]
From an environmental perspective, Methyl Mercaptan plays a significant role in odour pollution, particularly near industries such as paper mills, refineries, and certain chemical plants. When released into the air, even in small amounts, its potent smell can impact nearby communities, causing discomfort and potentially affecting air quality. Prolonged exposure in high concentrations can also lead to health concerns, such as irritation of the eyes, nose, and throat.
Methyl mercaptan is a by-product of asparagus in roughly 50% of humans and is responsible for the distinct change in urine odour (Richer et al., 1989).
The main chemical and physical properties of CH2O are as follows :
- Formula: CH3SH
- Molecular mass: 48.1
- Boiling point: 6°C
- Melting point: -123°C
- Relative density (water = 1): 0.9
- Flash point: Flammable gas
- Explosive limits, vol% in air: 3.9-21.8
CH3SH in Atmosphere:
Methyl mercaptan is naturally released into the atmosphere from microbial degradation of organic matter. It volatilizes naturally from soil and water. Once released in the atmosphere, it undergoes photooxidation. During the day, methyl mercaptan is oxidized by OH radicals, while during the night, it reacts with NO3 molecules in the atmosphere. As a result of these reactions, the effective lifetime of CH3SH in the atmosphere is about 1 to 26 hours.
2. Sources
Methyl Mercaptan (CH3SH) is naturally released from decaying plants, wetlands, and animal digestion. However, human activities like petrochemical production, paper manufacturing, and natural gas processing also add to its presence in the atmosphere.
It is used to manufacture oil, paper, plastics, jet fuel, and pesticides. Because of its strong rotten cabbage odour, it is also used as a chemical odorant to identify natural gas leaks. Other sources include sewage treatment plants, petroleum refining, starch manufacturing, wood-pulp mills, rendering plants, pharmaceutical industry, etc.
3. Permissible Exposure Limits for CH3SH
The permissible exposure limits of CH3SH in terms of continuous occupational exposure are given below:
|
OSHA (PEL) |
For general industry : 10 ppm Ceiling For construction industry : 0.5 ppm TWA For maritime : 0.5 ppm TWA |
|
ACGIH (TLV) |
0.5 ppm TWA |
|
NIOSH (REL) |
0.5 ppm Ceiling |
|
NIOSH (IDHL) |
150 ppm |
[Source: Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 15, National Research Council]
Permissible Exposure Limit (PEL) given by OSHA (Occupational Safety and Health Administration) defines the maximum concentration of CH3SH to which an unprotected worker may be exposed to. PEL may reference an eight-hour time-weighted average (TWA), a 15-minute short term exposure limit (STEL) or an instantaneous ceiling limit (CL) concentration that cannot be exceeded for any period of time. Similarly, Recommended Exposure Limit (REL) and Immediately Dangerous to Life of Health (IDLH) are the occupational exposure limit recommended by NIOSH (National Institute for Occupational Safety and Health) and the TLVs (i.e. threshold limit values) are the exposure guidelines given by ACGIH (American Conference of Governmental Industrial Hygienists)
The AEGL (Acute Exposure Guideline Levels) values by EPA (Environmental Protection Agency) of methyl mercaptan are given below. They represent threshold exposure limits (exposure levels below which adverse health effects are not likely to occur) for the general public and are applicable to emergency exposures ranging from 10 minutes (min) to 8 h.
Table: AEGL Values for methyl mercaptan (ppm)
|
Classification |
10 min |
30 min |
1 h |
4 h |
8 h |
|
AEGL-1 (non disabling) |
– |
– |
– |
– |
– |
|
AEGL-2 (disabling) |
40 |
29 |
23 |
14 |
7.3 |
|
AEGL-3 (lethal) |
120 |
86 |
68 |
43 |
22 |
[Source: https://www.epa.gov/aegl/methyl-mercaptan-results-aegl-program]
4. Health Impact of CH3SH
The EPA has declared methyl mercaptan a regulated toxic substance due to its adverse effects on human health. Exposure to humans can occur through eye/skin contact, inhalation, or ingestion, but ingestion is extremely unlikely due to the volatility of methyl mercaptan. Ingestion can irritate the mucous membranes, generating a burning sensation and excessive salivation. Low amounts will often irritate the conjunctiva. Conjunctivitis, photophobia, corneal bullae, weeping, discomfort, and blurred vision have all been documented as side effects of repeated low-concentration exposure. Skin contact might also irritate.
Humans are most commonly exposed to methanethiol by breathing. Exposure can result in fever, cough, shortness of breath, chest tightness and burning, pulmonary edema, respiratory failure, and collapse. It is possible to experience headaches, loss of smell, dizziness, a staggering gait, and heightened emotions. Memory loss, damage to the central and peripheral nerve systems, tremors, convulsions, and coma may also occur.
5. Possible Corrective Measures
The primary action is methyl mercaptan monitoring, i.e., measuring how much CH3SH concentrations you are exposed to. In addition to this, the following corrective measures can be taken:
- Use ventilation, local exhaust, or breathing protection.
- Wear safety goggles or eye protection in combination with breathing protection.
- Avoid going to or staying in low-lying areas where methyl mercaptan is produced, such as wetlands, dump sites, etc.
- Avoid staying upwind of wastewater treatment plants, sewage treatment plants, landfills, industrial areas of starch manufacturing, wood-pulp mills, etc.
- Always use appropriate personal protective equipment (PPE) while using CH3SH containing products or while going to places where high levels of CH3SH are suspected.
- Also, if high levels of CH3SH are detected, the area should be immediately vacant, and proper ventilation should be provided to remove the gas.
6. Measurement Methods of CH3SH Monitoring
Different working principles for methyl mercaptan monitoring in the ambient environment are chemiluminescence, semiconductor, and electrochemistry.
Chemiluminescence – This method of methyl mercaptan monitoring is based on the chemiluminescent reaction between methyl mercaptan (CH3SH) and ozone (O3). Based on this principle in the methyl mercaptan monitor, the CH3SH molecules present in the air sample react with ozone (produced using an ozone generator) to generate activated SO2. During the conversion, light energy at a specific wavelength is produced proportional to the amount of methyl mercaptan in the air sample. The light intensity is measured photometrically and converted to the CH3SH concentration.
Semiconductor – When a metal oxide semiconductor-based methyl mercaptan monitor is exposed to air sample, the CH3SH molecules react on the metal oxide surface of the sensor and dissociate into charged ions which alter the resistance of the film. This interaction is measured as a signal and is converted to the gas concentration. However, the energy consumption of such methyl mercaptan monitors is higher compared to others.
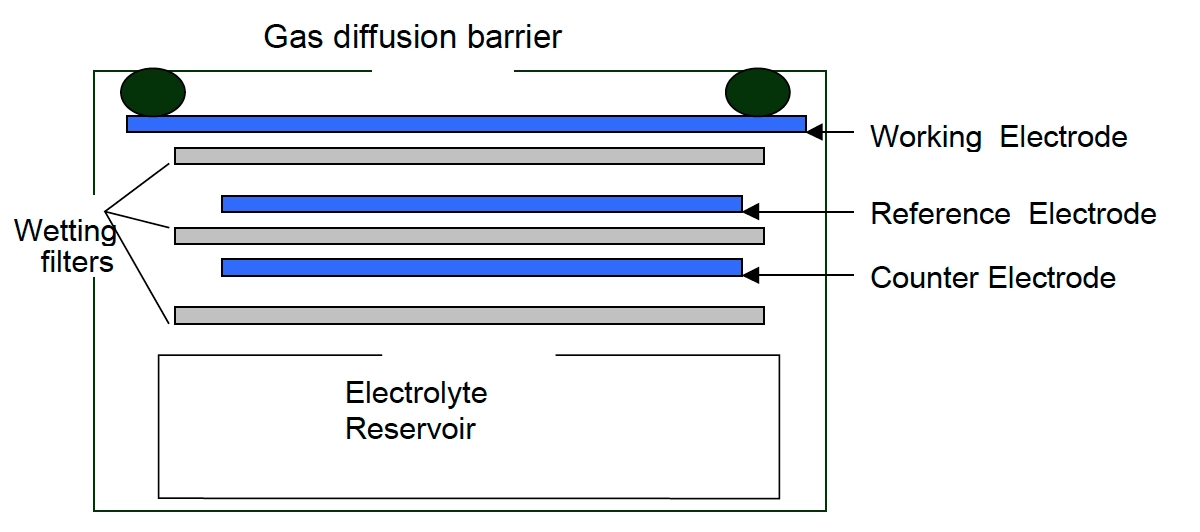
Electrochemical – CH3SH monitors working on the electrochemical principle are operated based on diffusion of methyl mercaptan gas into the sensor which results in the production of electrical signals proportional to the CH3SH concentration. It allows accurate measurement of even low concentrations of CH3SH, which is essential in methyl mercaptan monitoring in the ambient air.
[Source: iSCAPE Sensors Documentation]
7. Oizom’s Sensor Working Principle for CH3SH Monitoring
Oizom provides a range of methyl mercapton (CH3SH) sensor modules to monitor varying CH3SH levels based on your needs. Our sensors accurately measure CH3SH in ambient conditions, detecting concentrations in ppb/ppm. This sensor monitors carbon monoxide in real time. This sensor is integrated into a metal casing and ultra-low-noise support electronics, making it compact and reliable. This allows accurate gas detection even at very low concentrations in the atmosphere. This sensor works on the Electrochemical working principle to measure environmental air quality.
This sensor undergoes calibration using standard gases and tools to ensure high sensitivity and accuracy. Each gas sensor is calibrated with zero and span checks in a controlled lab, following Section 12.2 of the USEPA Quality Assurance Handbook for Air Pollution Measurement Systems Volume II. Calibration is done using NIST-traceable gas standards for reliable performance.
The Methyl mercaptan sensor module is used in Oizom’s odour and air quality monitoring systems, such as Odosense and AQBot. It is perfect for applications in sewage treatment plants, effluent treatment plants, industrial settings, landfills, and solid waste management facilities.
8. Why Choose Oizom CH3SH Sensor?
- Compact: Our sensors are small and easy to install, perfect for use in any space, making them ideal for portable air quality monitoring. The CH3SH sensors come pre-calibrated and can be quickly replaced in just a few minutes by removing and replacing the old sensor with a new one.
- Durable: The CH3SH sensor has a long life of almost two years.
- Energy Efficient: There is no need to be concerned about energy usage because our sensors are not only accurate but also energy efficient. Powered up with just 3.3 to 5V for efficient, reliable performance!
- In-house sensor tech: Oizom applies advanced data processing algorithms to compensate for the effects of temperature and humidity on the sensor output. The algorithm is designed to automatically update based on weather conditions and seasonal changes, removing its influence on the sensor performance. The advanced algorithms also compensate for the effect of the cross-sensitive gas.
- Ultra-Low Noise Electronics: The sensor module’s design is crucial for accurate measurements. Each sensor is housed in a metal casing with ultra-low-noise electronics and a base PCB. The metal casing shields the sensor and electronics from electromagnetic interference, preventing false readings.
- RoHS Compliant: Our sensors comply with the RoHS criteria for restricting hazardous substances in electrical and electronic devices.
9. Reasons Why CH3SH Monitoring is Important
- Methyl mercaptan is a colorless, highly flammable, toxic gas with a distinct, strong, unpleasant odour released from microbial degradation of organic matter.
- It is an organosulfur volatile organic compound (VOC) that can be easily ignited and decomposed, emitting highly toxic fumes and flammable vapors.
- Exposure to methyl mercaptan irritates the eyes, skin as well as the respiratory tract, causes respiratory distress, damages the liver and kidneys, and causes respiratory paralysis, leading to coma and death.
- Prolonged exposure of the CH3SH can quickly deaden a person’s sense of smell, making the odour of methyl mercaptan an unreliable indicator of its presence. Hence, other means, such as using methyl mercaptan monitors, are viable solutions to provide adequate warning of hazardous exposure.
- Real-time monitoring of CH3SH levels helps determine their source and formulate an action plan to control methyl mercaptan emissions.
FAQs
1. What is Methyl Mercaptan (CH3SH)?
CH3SH is a colorless gas with a strong, foul odour, often linked to natural decay and industrial emissions.
2. How does CH3SH affect the environment?
It causes odour pollution and can reduce air quality, impacting ecosystems and communities.
3. Which industries release CH3SH?
Industries like petrochemicals, paper mills, and natural gas processing are major contributors.
4. Is CH3SH harmful to humans?
High concentrations can irritate the eyes, nose, and throat and cause headaches.
5. How can CH3SH emissions be controlled?
Effective air quality monitoring helps detect and manage emissions, protecting the environment and public health.
6. What does mercaptan smell like?
Mercaptan smells like rotten cabbage, eggs, or sewer gas. It’s added to odourless natural gas to detect leaks.
7.What should I do if I smell methyl mercaptan?
Ventilate the area, evacuate if the smell persists, notify others, and contact emergency services. Do not try to find the leak on your own.
8. How can I reduce methyl mercaptan emissions?
Compost food scraps, support proper waste treatment, choose eco-friendly products, and raise awareness about its impact on air quality.
9.How do governments regulate methyl mercaptan emissions?
Governments set emission limits, enforce regulations through audits, invest in green tech, and provide information on exposure risks.