Ammonia (NH3) is a common air pollutant that many people might not realize has significant environmental implications. Despite its absence from numerous global air quality criteria, including those of the World Health Organization, ammonia still plays a crucial role in both nature and industry. This colorless gas, known for its distinct, pungent smell, is naturally produced by living organisms and is essential for plant growth and soil health as part of the nitrogen cycle. However, when not managed properly, ammonia can contribute to environmental challenges like air and water pollution. Understanding ammonia, its uses, and its environmental impact is essential for making informed decisions about interacting with this powerful compound in our daily lives and industries.
Let’s dive into the world of ammonia and explore its significance in nature and human activities. This article covers information on ammonia, its sources in the ambient air, permissible levels, health and environmental impact, possible corrective measures, the need for ammonia monitors, and different methods of NH3 monitoring.
1. What is NH3?
Ammonia (NH3) is a poisonous gas with one nitrogen and three hydrogen atoms. It occurs naturally in trace levels but can also be generated through industrial processes. Ammonia is used in the production of fertilizers, refrigeration, and cleaning products. It is used as a raw material to produce compounds like nitric oxide. Ammonia is a colorless gas at room temperature with a very pungent, irritating smell detectable at the concentration level of 25 ppm in the atmosphere.
Ammonia (NH3) is a toxic gas that can be irritating to the eyes, nose, throat, and respiratory tract. Inhaling large quantities of NH3 can be fatal. This highly reactive, corrosive, alkaline gas dissolves easily in water to form ammonium hydroxide. While it’s not highly flammable, it can explode when exposed to high heat. These potential dangers underscore the need for caution and awareness when dealing with ammonia.
Ammonia in Atmosphere:
NH3 is the most abundant alkaline gas in the atmosphere, released naturally from soil from bacterial processes and decaying organic matter, including plants, animals, and animal wastes.
In the atmosphere, ammonia reacts with acid pollutants, such as the products of SO2 and NOx emissions, to produce fine aerosols containing ammonium ions (NH4+). While the lifetime of NH3 is relatively short (<10-100 km), NH4+ may be transferred much longer distances (100->1000 km) (Asman et al. 1998, Fowler et al. 1998).
The transformation of ammonia gas in the atmosphere is based on the chemical reaction with acidic gas species that form NH4+ salt aerosols, also known as secondary inorganic PM2.5. Gaseous ammonia first reacts with sulfuric acid (H2SO4) to form ammonium sulfate ((NH4)2SO4), and if more amount of ammonia is available, it then reacts with nitric acid (HNO3) to form ammonium nitrate (NH4NO3). These reactions are highly dependent on the atmospheric concentration of ammonia (NH3), nitrogen oxides (NOx), sulfur oxides (SOx), temperature and humidity.
In addition to air pollution, Ammonia in the air, when absorbed by the water, contributes to the acidity, salinization, and oxidation of ammonium salts in rivers, which affects marine life. Due to lower SOx emissions in urban areas, ammonium nitrate formed by reaction with HNO3 is the major source of fine particulate matter, specifically released from agricultural sources.
2. Sources
The primary sources of ammonia are agricultural processes, specifically fertilizer manufacturing and livestock waste management. Animal dung, which contains NH3, is combined with other useless organic matter, such as hay and water runoff, to create a slurry. This combination is allowed to sit until it turns into a natural fertilizer. Ammonia is naturally produced as a byproduct of this process. As a result, these agricultural operations cause a significant increase in NH3 concentrations in the spring. Agriculture is estimated to account for 80% to 95% of ammonia emissions in developed countries, which then react with the H2SO4 mentioned above from SO2 and HNO3 from NOx to produce PM. In fact, agricultural ammonia accounts for up to 50% of particulate matter in European cities and 30% (US) of PM2.5 air pollution. (Katie et al., 2022)
It is also found in many household and industrial-strength cleaning products.
- Vehicular Emissions: Vehicular NH3 emissions are co-emitted with nitrogen oxides (NOx) and may have a more effective pathway to particle formation in urban environments than NH3 from agricultural activities, which tend to be emitted in rural, low-NOx regions. The burning of gasoline emits 0.30-0.47 g/kg of ammonia into the atmosphere. Diesel combustion contributes 0.34–0.50 g/kg. (Qijun et al., 2020).
Two main sources of NH3 emissions from road vehicles are catalyst-equipped gasoline vehicles and light- and heavy-duty diesel vehicles that rely on selective catalytic reduction (SCR).
- Sewage Treatment Plant: Ammonia in wastewater refers to nitrogen in the form of free ammonia and ionic ammonium, which is mostly produced by the decomposition of nitrogen-containing organic matter in residential sewage, ammonia synthesis, and other industrial wastewater, as well as agricultural drainage.
The oxidation of NH4+-N reduces the concentration of dissolved oxygen in water, resulting in dark and smelly water and a reduction in water quality, which affects the survival of aquatic animals and plants.
Processing of sewage waste can produce a high amount of ammonia. If not monitored and controlled, it can be fatal.
- Basics of Ammonia Production: Ammonia (NH3) is an important chemical used to make fertilizers, plastics, dyes, and many other products.
- The Haber-Bosch process, which transforms hydrogen and nitrogen into ammonia, could be one of the most significant commercial chemical reactions ever developed. The technology made ammonia fertilizer widely available, contributing to a global population boom by increasing agricultural yields rapidly in a short period.
Globally, ammonia production plants made 157.3 million metric tons (t) of the compound in 2010, according to the Institute for Industrial Productivity’s Industrial Efficiency Technology Database. Between 75 and 90% of this ammonia goes toward making fertilizer, and about 50% of the world’s food production relies on ammonia fertilizer.
- Ammonia in various Industries: Ammonia is vital in many industries due to its versatility. In agriculture, it’s used in fertilizers to boost crop yields. The pharmaceutical industry uses ammonia in drug production, while it also serves as a coolant in refrigeration and is key in making textiles and cleaning agents. In petrochemicals, ammonia is a precursor for products like urea, ammonium nitrate, and plastics. It also helps reduce sulfur emissions in industrial processes through flue gas desulfurization.
3. Permissible Exposure Limits for NH3
The breakpoint concentrations describing air quality based on the ammonia concentrations derived in India are given below. Here, the daily average NH3 levels in the ambient air of up to 400 µg/m3 (i.e., 0.574 ppm) are considered satisfactory.
Table: Breakpoints of NH3 (µg/m3) – 24hr in India
|
AQI Category |
Breakpoint concentration |
|
Good (0-50) |
200 |
|
Satisfactory (50-100) |
400 |
|
Moderately polluted (101-200) |
800 |
|
Poor (210-300) |
1200 |
|
Very poor (301-400) |
1800 |
|
Severe (401-500) |
1800+ |
[Source: National Ambient Air Quality Index, CPCB (Oct 2014)]
Apart from ambient limits, the permissible exposure limits of NH3 in terms of continuous occupational exposure indoors are given below:
|
8-hr. TWA |
STEL |
Ceiling Limit |
|
|
Federal OSHA PEL |
50 ppm |
NA |
300 ppm |
|
NIOSH REL |
25 ppm |
35 ppm |
300 ppm |
|
ACGIH TLV (2010) |
25 ppm |
35 ppm |
300 ppm |
Permissible Exposure Limit (PEL) given by OSHA (Occupational Safety and Health Administration) defines the maximum concentration of NH3 to which an unprotected worker may be exposed. PEL may reference an eight-hour time-weighted average (TWA), a 15-minute short-term exposure limit (STEL), or an instantaneous ceiling limit (CL) concentration that cannot be exceeded for any period. Similarly, the Recommended Exposure Limit (REL) is the occupational exposure limit recommended by NIOSH (National Institute for Occupational Safety and Health), and the TLVs (i.e., threshold limit values) are the exposure guidelines given by ACGIH (American Conference of Governmental Industrial Hygienists).
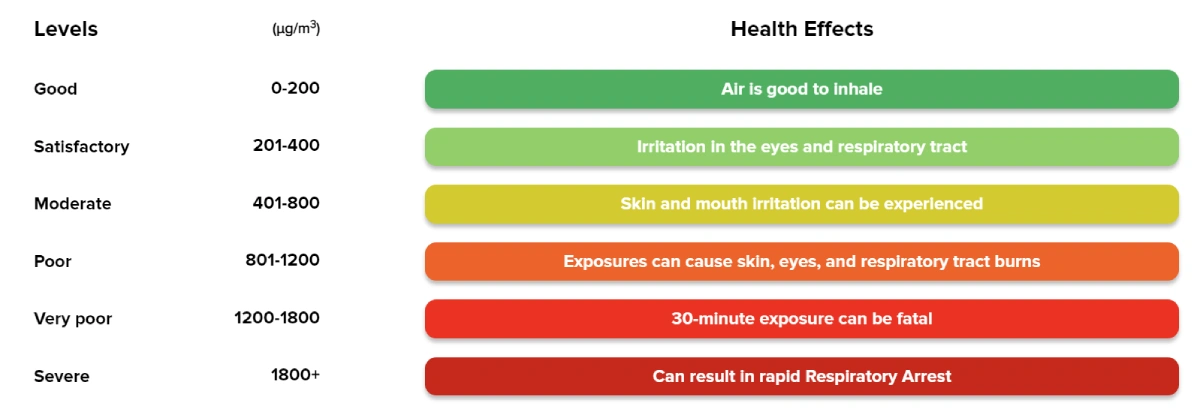
Ammonia Concentrations and Health Effects:
Know the risks. Stay informed on how different levels of ammonia exposure impact your health.
Source – NAQI as per CBCB. 2-h hourly average values
The above table describes the ammonia concentration in (µg/m3) with its health effects.
4. Indicative Ammonia Limit Values for Occupational and Facility Safety
There are recommended indicative occupational exposure limit values for Ammonia exposure, which vary depending on the country.
|
Ammonia CAS No: 7664-41-7 |
Long-term – 8-hour (TWA) |
Short-term – 15-minute (STEL) |
||
|
ppm |
mg/m3 |
ppm |
mg/m3 |
|
|
European Union |
20 |
14 |
50 |
36 |
|
France |
10 |
7 |
20 |
14 |
|
Germany |
20 |
14 |
40 |
28 |
|
Italy |
20 |
14 |
50 |
36 |
|
Spain |
20 |
14 |
50 |
36 |
|
United Kingdom |
25 |
18 |
35 |
25 |
|
Turkey |
20 |
14 |
50 |
36 |
5. Health & Environmental Impact of NH3
Health Impact
Ammonia gas can harm an individual’s health because it is irritating and poisonous. Thus, taking necessary care when handling it is critical to avoid exposure and protect human safety. Following are some common health hazards one can experience if exposed to ammonia gas:
- When inhaled at high levels, NH3 is a harmful gas that irritates the respiratory tract and eyes. Additionally, Even at lower concentrations, it can cause throat and skin irritation.
- High levels of ammonia exposure may cause poisoning, resulting in symptoms such as confusion, dizziness, and seizures, and can even be fatal in severe cases.
- Chronic exposure to low levels of ammonia can potentially have long-term health consequences, including chronic bronchitis, asthma, and lung damage.
- Ingestion of liquid ammonia can lead to digestive issues such as nausea, vomiting, and abdominal pain.
- Children are more vulnerable to NH3 exposure because of their larger lung surface area relative to body weight. Their shorter height also makes them more likely to inhale NH3 vapors that stay near the ground.
The toxic effects associated with exposure to NH3 concentration is given below:
|
NH3 (ppm) |
Effect on human health |
|
50 |
Irritation to eyes, nose, and throat (2 hours’ exposure) |
|
100 |
Rapid eye and respiratory tract irritation |
|
250 |
Tolerable by most people (30 – 60 minutes’ exposure) |
|
700 |
Immediately irritating to the eyes and throat |
|
>1500 |
Pulmonary edema, coughing, laryngospasm |
|
2500-4500 |
Fatal (30 minutes’ exposure) |
|
5000-10,000 |
Rapidly fatal due to airway obstruction may also cause skin damage |
[Source from Ammonia Toxicological Overview, Public Health England]
Environmental Impact
Ammonia pollutes terrestrial and aquatic habitats, causing eutrophication and acidification. When used as fertilizer, the nitrogen in NH3 can seep into the soil, raising its acidity and negatively impacting plant growth. Additionally, NH3 can evaporate into the surrounding air, contributing to air pollution and forming harmful particulate matter (PM2.5). This can reduce air quality and pose health risks to nearby communities and ecosystems. It is hazardous to aquatic organisms; contaminant accumulation in their bodies causes death. Increased nitrogen levels also induce algal overgrowth, which blocks light and deprives plants of nutrition, resulting in their demise.
Plants like lichens and mosses are especially sensitive to nitrogen levels. Even a modest rise can be fatal. This has an impact on the surrounding wildlife, which is dependent on these species for survival.
Formation of Secondary Inorganic Aerosols (SIA): Ammonia reacts with H2SO4 and HNO3 present in the atmosphere to form ammonium salts that remain in the atmosphere for a few days up to a week as the particulate matter before depositing back to the ground, impacting human health and environment over large scales.
Be cautious that ammonia vapors are harmful to cattle. Dairy, swine, and poultry livestock producers operating near or downwind of a release or potential release must be notified so that necessary action can be taken.
6. Possible Corrective Measures
The primary action is NH3 monitoring, i.e., to measure how much NH3 concentrations you are exposed to. In addition to this, the following corrective measures can be taken:
- Avoid going to or staying in low-lying areas where NH3 is produced or used, such as agricultural or poultry farms.
- To avoid exposure, workers handling ammonia gas should use suitable personal protection equipment (PPE), such as respirators, goggles, gloves, and protective clothes.
- Facilities handling ammonia gas should have adequate ventilation systems to prevent harmful quantities of ammonia gas from accumulating in the air.
- Ammonia gas is classified as a hazardous material for transportation, and strict regulations apply to its transport by road, rail, or sea.
- Equipment and facilities handling ammonia gas should be regularly inspected and maintained to prevent leaks or other safety hazards.
- Facilities handling ammonia gas must comply with applicable safety laws and standards, such as those established by OSHA, the EPA, or municipal authorities.
7. Measurement Methods of NH3 Monitoring
Different working principles for ammonia monitoring in the ambient environment are chemiluminescence, semiconductor, and electrochemistry.
Chemiluminescence – Chemiluminescence method-based NH3 monitors continuously measure NH3 and NOx with just some modifications. In the NH3 monitor, a thermal NH3 converter module is placed upstream of the conventional chemiluminescence NOx analyzer, where the NH3 and NO2 are transformed to NO in one part of the air stream. In contrast, the other part contains the original NO content. Both the streams are passed to react with Ozone (O3), where the light produced by the chemiluminescent reaction of O3 and NO is measured photometrically and recorded. The ammonia monitor subsequently detects the total NOx after being passed through NH3 scrubbers in a separate channel, such that the difference in both signals is proportional to the total NH3 concentration in the air sample. For the NH3 monitors based on this principle, specific precautions should be taken to address the interferences caused by other organic nitrogen compounds that can transform to NO, resulting in inaccurate results.
Semiconductor – When a metal oxide semiconductor-based ammonia monitor is exposed to an air sample, the NH3 molecules react on the metal oxide surface of the sensor and dissociate into charged ions, altering the film’s resistance. This interaction is measured as a signal and is converted to the gas concentration. However, such ammonia monitors’ energy consumption is higher than other monitors.
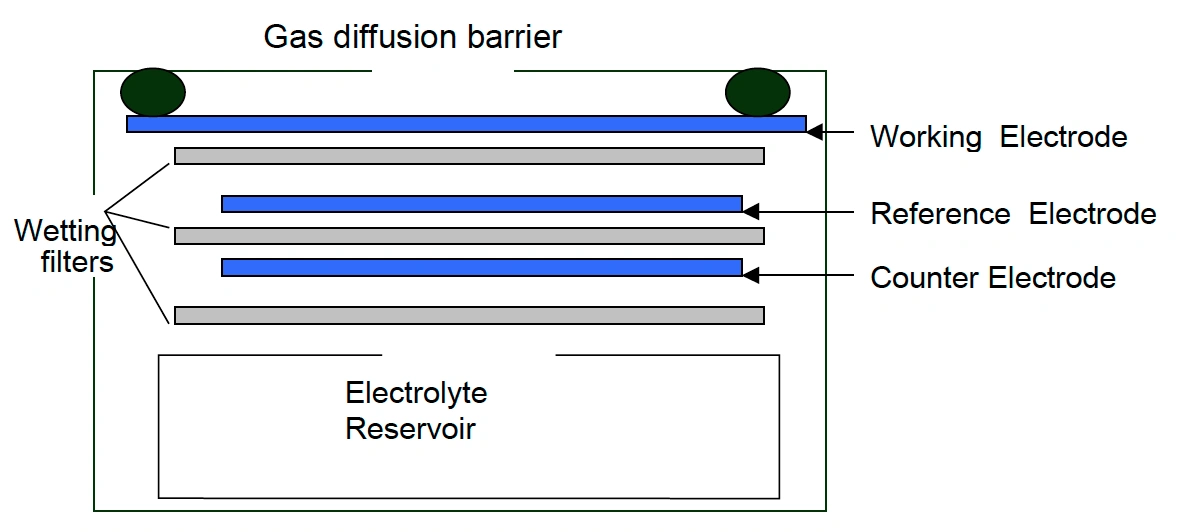
Electrochemical – NH3 monitors working on the electrochemical principle are operated based on the diffusion of ammonia gas into the sensor, producing electrical signals proportional to the NH3 concentration. It allows accurate measurement of even low concentrations of NH3, which is essential in NH3 monitoring in the ambient air.
[Source: iSCAPE Sensors Documentation]
8. Oizom’s Sensor Working Principle for NH3 Monitoring
Oizom provides a range of Ammonia (NH3) sensor modules to monitor varying NH3 levels based on your needs. Our sensors accurately measure NH3 in ambient conditions, detecting concentrations in ppb/ppm.. This sensor monitors Ammonia in real time. This sensor is integrated into a metal casing and ultra-low-noise support electronics, making it compact and reliable. This allows accurate gas detection even at very low concentrations in the atmosphere. This sensor works on the Electrochemical working principle to measure environmental air quality.
This sensor undergoes calibration using standard gases and tools to ensure high sensitivity and accuracy. Each gas sensor is calibrated with zero and span checks in a controlled lab, following Section 12.2 of the USEPA Quality Assurance Handbook for Air Pollution Measurement Systems Volume II. Calibration is done using NIST-traceable gas standards for reliable performance.
The NH3 sensor module is integrated into outdoor air quality monitoring systems like AQBot and Odosense. It is ideal for sewage, effluent treatment, industries, landfills, solid waste management, and air quality research projects. By utilizing this sensor module, users can ensure they receive accurate, real-time data on NH3 levels, aiding in effectively monitoring and managing air quality across diverse environments.
9. Why Choose Oizom NH3 Sensor?
- Compact: Our sensors are small and easy to install, perfect for use in any space, making them ideal for portable air quality monitoring. The NH3 sensors come pre-calibrated and can be quickly replaced in just a few minutes by removing and replacing the old sensor with a new one.
- Durable: The NH3 sensor has a long life of almost two years.
- Energy Efficient: There is no need to be concerned about energy usage because our sensors are accurate and energy efficient. Powered up with just 3.3 to 5V for efficient, reliable performance!
- In-house sensor tech: Oizom applies advanced data processing algorithms to compensate for the effects of temperature and humidity on the sensor output. The algorithm is designed to automatically update based on weather conditions and seasonal changes, removing its influence on the sensor performance. The advanced algorithms also compensate for the effect of the cross-sensitive gas.
- Ultra-Low Noise Electronics: The sensor module’s design is crucial for accurate measurements. Each sensor is housed in a metal casing with ultra-low-noise electronics and a base PCB. The metal casing shields the sensor and electronics from electromagnetic interference, preventing false readings.
- RoHS Compliant: Our sensors comply with the RoHS criteria for restricting hazardous substances in electrical and electronic devices.
10. Reasons Why NH3 Monitoring is Important
Simple actions can reduce ammonia levels, but they must be applied nationwide.
- NH3 is a very soluble, colorless gas with a strong, pungent smell primarily released from decaying organic matter, including animal wastes and fertilizer use.
- NH3 forms secondary particulate matter of ammonium salts (NH4+) by reacting with the acids of SOx and NOx, which move large distances along the air, impacting human health and the environment of both local and international (transboundary) scales.
- NH3, on contact or when inhaled, rapidly reacts with the moisture-containing parts of the body and causes irritation and damage to the cells of the eyes, nose, throat, and respiratory tract. Additionally, it plays a significant role in atmospheric nitrogen deposition in sensitive ecosystems, causing acidification and eutrophication of soils and natural waters.
- Ammonia gas levels in the atmosphere must be monitored frequently, particularly in industrial and agricultural contexts where it is commonly employed. This can reduce exposure to hazardous ammonia gas quantities while protecting the environment.
- Monitoring ammonia gas in the atmosphere is critical for protecting human health, the environment, and regulatory compliance. Regular monitoring can assist in identifying sources of ammonia emissions and executing reduction actions, resulting in a safer and healthier environment for all.
- Real-time monitoring of NH3 levels helps calculate the air quality index to deliver health advisories and formulate an action plan to meet standards.
FAQs
What is ammonia (NH3)?
Ammonia (NH3) is a colorless gas with a strong, pungent odour commonly used in agriculture, refrigeration, and manufacturing.
How does ammonia affect the environment?
Ammonia can contribute to air and water pollution, harm aquatic ecosystems, and form fine particulate matter that impacts air quality.
What are the health risks of ammonia exposure?
Inhalation of ammonia gas can irritate the respiratory system, eyes, and skin. High concentrations can cause lung damage and serious health issues.
What are the main sources of ammonia in the atmosphere?
Ammonia emissions primarily come from agricultural activities like livestock waste, fertilizer use, and industrial processes.
How can ammonia levels be monitored?
Ammonia levels can be monitored using Oizom devices, which utilize NH3 gas sensors based on the electrochemical principle. Other methods include chemical test kits or electronic monitoring systems to ensure safe air quality.