Nitrogen dioxide (NO₂) is a critical air pollutant formed during high-temperature combustion processes when atmospheric nitrogen (N₂) reacts with oxygen. As a major byproduct of vehicular emissions, power generation, and industrial activities, NO₂ plays a pivotal role in urban air pollution. It is a precursor to ground-level ozone (O₃) and fine particulate matter (PM₂.₅), both of which contribute to respiratory diseases and environmental degradation.
Regulated under the Clean Air Act as one of the six criteria pollutants, NO₂ is closely monitored to ensure compliance with air quality standards. Its impact on air quality extends beyond direct inhalation risks; it also drives photochemical smog formation, exacerbating health issues for vulnerable populations, particularly those with asthma or pre-existing respiratory conditions.
Given its atmospheric reactivity and short-lived nature, precise NO₂ monitoring is essential for air quality management. Advanced measurement techniques, including chemiluminescence analyzers, optical spectroscopy, and electrochemical sensors, enable accurate detection and regulatory compliance. This article covers nitrogen dioxide (NO₂) in the air, its sources, permissible levels in ambient air, health and environmental impacts, possible corrective measures, the need for NO₂ monitors, and different methods of NO₂ monitoring. It provides a comprehensive understanding of a critical factor in air quality.
1. What is Nitrogen Dioxide (NO₂)?
Gas Characteristics
- Physical State: Yellowish-brown liquid at low temperatures; reddish-brown gas above 70°F (21°C).
- Odour: Strong, pungent, and acrid.
- Density: Heavier than air, allowing it to accumulate in low-lying areas.
- Health Risks: Fatal if inhaled in high concentrations; causes severe respiratory irritation and lung damage.
- Reactivity: Oxidizers can intensify fires and react with combustible materials.
- Water Reaction: Forms nitric acid (HNO₃) when combined with water, contributing to acid rain.
- Stability: Stored as a pressurized gas, with the risk of explosion if heated.
- Other Names: Nitrogen dioxide, NO₂, nitrogen oxide, nitrogen peroxide, dinitrogen tetroxide, and nitrito.
- CAS Number: 10102-44-0.
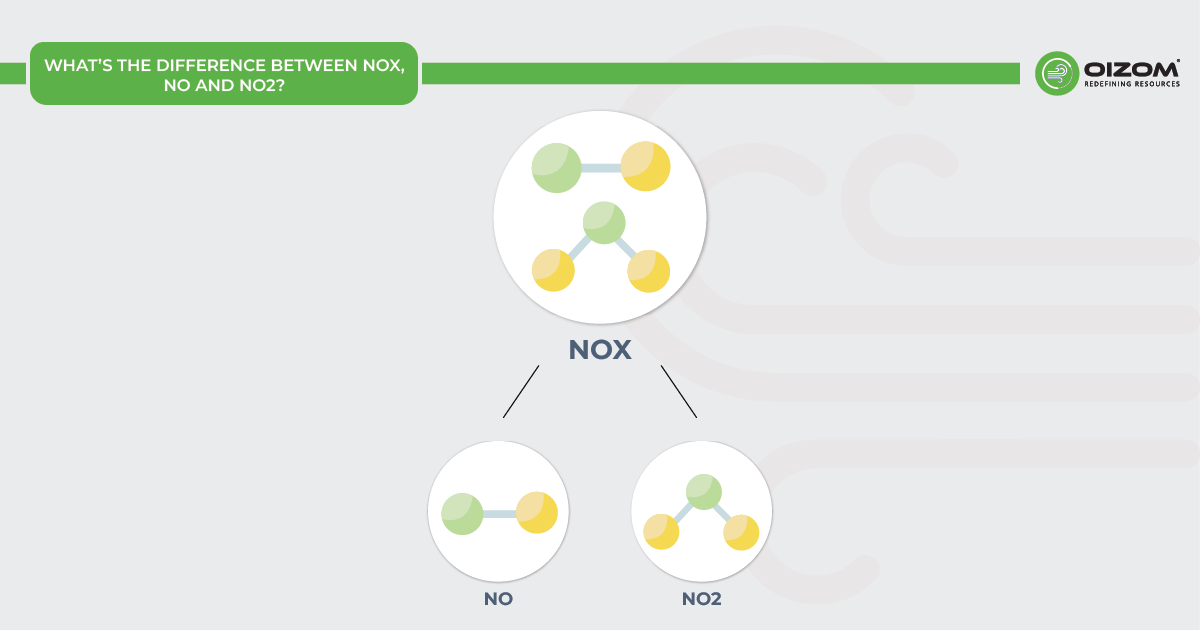
2. What’s the Difference Between NOX, NO and NO2?
- The phrase “nitrogen oxides” (NOx) refers to both nitrogen dioxide (NO2) and nitrogen monoxide (NO).
- Nitrogen monoxide (NO) is a colorless gas and one of the primary oxides of nitrogen.
- Nitrogen dioxide (NO2) is a reddish-brown gas with a unique, acidic odour. It is one of several nitrogen oxides.
Nitric Oxide: Nitrogen monoxide (NO), often known as nitric oxide, is a colorless gas with a strong, sweet odour and a hazardous air pollutant. It consists of one nitrogen atom bonded to one oxygen atom. Due to one unpaired electron, it is highly reactive and thus rapidly oxidized (within a few minutes) to NO2.
Nitrogen Dioxide: Nitrogen dioxide (NO2) is a critical air pollutant highlighted by air quality guidelines like those from the World Health Organization. NO2 is a reddish-brown acidic gas with a pungent, dissolves in water, and acts as a strong oxidant and corrosive. It forms when nitric oxide (NO) oxidizes during combustion, such as in diesel engines and power plants burning coal, oil, gas, wood, or waste. NO2 gas itself is not flammable. However, it can accelerate the burning of combustible materials.
Nitrogen Dioxides in the Atmosphere:
Nitrogen dioxide (NO₂) plays a crucial role in atmospheric chemistry and air quality. It is primarily introduced into the atmosphere through combustion processes, such as vehicular emissions, industrial activities, and power generation. Once emitted, NO₂ undergoes complex photochemical reactions, contributing to ozone (O₃) formation, particulate matter (PM₂.₅) generation, and acid deposition.
Of the nitrogen compounds released during combustion, 10% is NO2, while 90% is NO. Once released in the atmosphere, Nitrogen dioxide can combine with other atmospheric chemicals to generate acid rain, harming ecosystems and human-built monuments, structures, and cultural sites. Nitrogen oxides, including NO₂, produce nitrate particles that cause haze and reduced visibility. Elevated NO₂ levels can cause environmental damage, including nutrient pollution in coastal waters.
Atmospheric Chemistry of NO₂
In sunlight, NO₂ participates in the photolytic cycle, influencing ozone production:
- NO2+hν→NO+O (wavelength < 400 nm)
- O+O2→O3
This reaction leads to tropospheric ozone formation, a key component of smog, particularly in urban areas with high vehicle density. NO₂ also reacts with hydroxyl radicals (OH•) and volatile organic compounds (VOCs) to produce secondary pollutants such as peroxyacetyl nitrates (PANs), which contribute to long-range pollution transport.
The atmospheric chemistry in the areas where vehicular emission is a predominant source generally follows the trend given below:
- There is often a higher concentration of NO2 during peak traffic times.
- The concentration of O3 increases during the day as the sunlight rises, while the NO2 concentration decreases. This happens because NO2 reacts in the presence of the sun to form O3.
- In the late evening, NO2 concentrations build up as no sunlight converts NO2.
Transformation and Deposition
- NO₂ reacts with water (H₂O) to form nitric acid (HNO₃), a major component of acid rain, which degrades soil, damages vegetation, and acidifies water bodies.
- It can combine with ammonia (NH₃) and other compounds to form secondary aerosols, increasing PM₂.₅ concentrations, which pose severe health risks.
- NO₂ has a short atmospheric lifetime (a few hours to a day) before being removed via wet and dry deposition, affecting local and regional air quality.
3. Sources of NO2
NO₂ is prevalent in the environment in small quantities, but due to our excessive use of resources and other activities, nitrogen dioxide levels are rising to a dangerous level. Some of the common Natural and man-made sources of NO₂ are listed below:
- Vehicle Emissions – The most significant source of NO₂ pollution, mainly from cars, trucks, and buses.
- Industrial Activities – The second-largest contributor, including manufacturing and heavy industries.
- Power Plants & Thermal Stations – Burning coal, oil, or gas releases NO₂ into the air.
- Wastewater Treatment Plants – Certain treatment processes generate NO₂ emissions.
- Petroleum Refining – NO₂ is released during oil extraction and refining.
- Pulp and Paper Industry – Some chemical processes used in paper production emit NO₂.
- Diesel-Powered Equipment – Construction and farming machinery using diesel fuel produce NO₂.
- Agriculture – Fermentation in silos releases NO₂, particularly in enclosed storage.
- Chemical Industry – NO₂ is used in polymer production to prevent unwanted reactions and in chemical synthesis for making nitrates, rocket fuel, and even flour bleach.
- Indoor Sources – Gas stoves and kerosene heaters can generate harmful NO₂ levels inside homes.
Did you know this? In 2020, human-made sources in the U.S. emitted 7.64 million short tons of nitrogen oxides (down from 15 million short tons per year in 2011), mainly from burning fuels.
4. Permissible Levels of NO2
Below are the breakpoint concentrations describing air quality based on nitrogen dioxide concentrations for different countries. In India, daily NO2 levels of up to 80 µg/m3 are considered satisfactory.
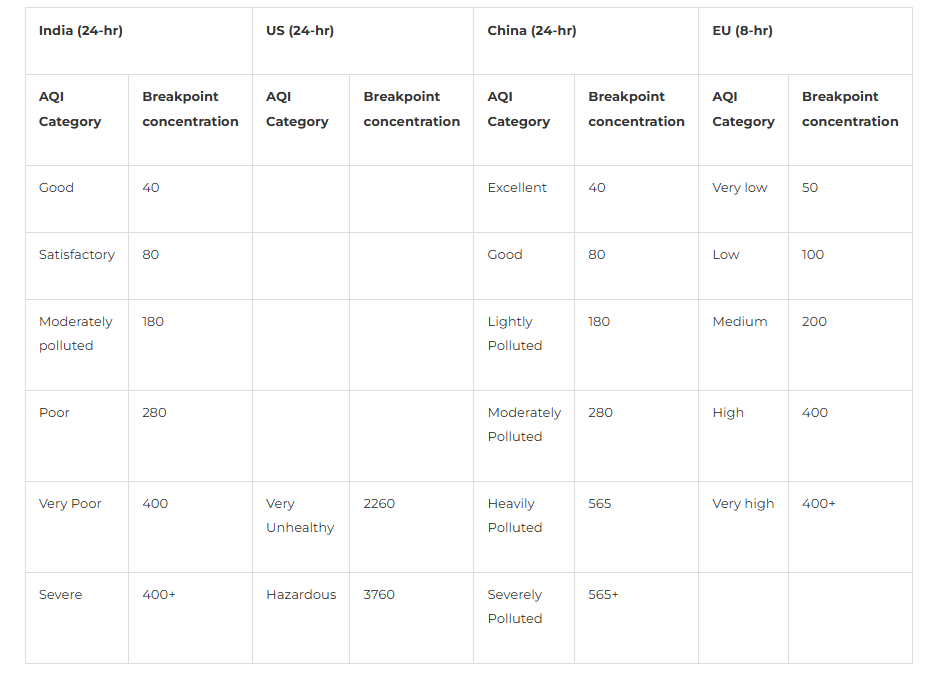
[Source: National Ambient Air Quality Index, CPCB (Oct 2014)]
The World Health Organization (WHO) has updated its air quality guidelines to better protect public health. The new NO2 guideline is set at 10 µg/m³ as an annual average, marking a significant reduction of 30 µg/m³ from the previous guideline established in 2005. This update underscores the WHO’s commitment to enhancing global air quality standards to safeguard public health.
The table below will represent WHO Indoor and Outdoor Air Quality Guidelines for the NO2
| Source | Indoor Air Quality | Outdoor Air Quality |
|---|---|---|
| NO2 | 1 Hour = 200 µg/m3 Annual = 40 µg/m3 | 24 Hour = 25 µg/m3 Annual = 10 µg/m3 |
Source – National Library of Medicine
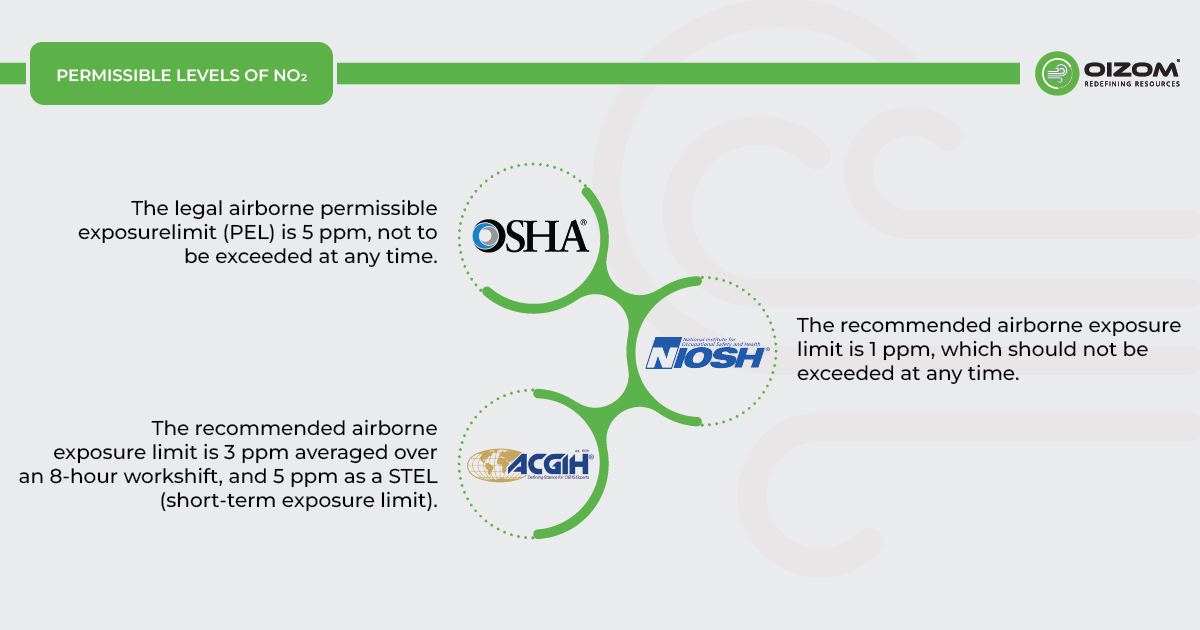
WORKPLACE EXPOSURE LIMITS for NO2
- OSHA: The legal airborne permissible exposure limit (PEL) is 5 ppm, not to be exceeded at any time.
- NIOSH: The recommended airborne exposure limit is 1 ppm, which should not be exceeded at any time.
- ACGIH: The recommended airborne exposure limit is 3 ppm averaged over an 8-hour workshift, and 5 ppm as a STEL (short-term exposure limit).
*Nitrogen Dioxide may cause mutations. All contact with this chemical should be reduced to the lowest possible level
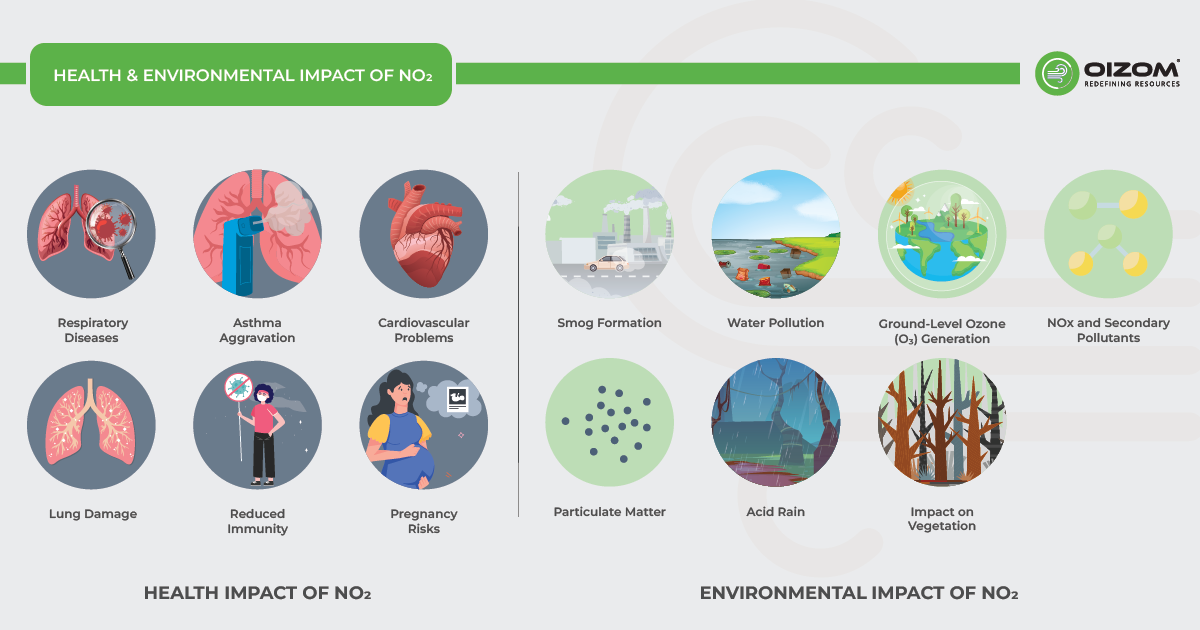
5. Health & Environmental Impact of NO2
NO2 interacts with O2 to produce nitric acid, which causes corrosion and contributes to the development of other pollutants such as smog, PM, and acid rain. It is a flame accelerator but is not flammable.
Health Impact
Nitrogen dioxide (NO₂) is a highly reactive gas that significantly affects respiratory and cardiovascular health. Due to its acidic nature and ability to penetrate deep into the lungs, exposure to NO₂ can lead to several serious health conditions:
- Respiratory Issues: NO₂ irritates the airways, causing breathing difficulties, coughing, wheezing, and reduced lung function.
- Lung Damage: Long-term exposure can result in altered lung function, increasing the risk of chronic respiratory diseases.
- Asthma Aggravation: NO₂ is a known trigger for asthma attacks, particularly in children and individuals with pre-existing lung conditions.
- Reduced Immunity: Prolonged exposure weakens the immune system, making individuals more vulnerable to respiratory infections.
- Cardiovascular Effects: High NO₂ levels are linked to heart failure and other cardiovascular diseases due to increased oxidative stress and inflammation.
- Pregnancy Risks: Studies suggest that exposure to elevated NO₂ concentrations may contribute to low birth weight and a higher risk of premature death.
The mental health concerns investigated included common mental disorders (CMD), sleep apnea, anxiety, depression, and suicide.
Were you aware of this? A 2022 review of multiple studies found that high levels of NO₂, along with particulate matter and sulfur dioxide, were strongly linked to heart and lung damage. They also affected pregnancy and birth outcomes and were likely connected to a higher risk of kidney and nerve damage, autoimmune diseases, and cancer.
It should be noted that nitrogen dioxide concentrations in indoor air can increase due to human activities such as heating and cooking, both of which emit nitrogen dioxide. If the indoor air is poorly ventilated, the gas can build up, causing very harmful effects to people who spend time indoors daily.
Below are Stats for the US, which show economic and health impact in the US
A study specifically examining the impacts of nitrogen dioxide exposure found that in areas with just a 5.9 parts per billion increase in pollutant concentration, there was
- A 22% increase in emergency room costs
- A 5% increase in outpatient costs,
- A 7% increase in annual direct healthcare costs.
Environmental Impacts:
Excessive NO₂ concentrations contribute to severe environmental degradation, affecting air, water, and plant life:
- Smog Formation: NO₂ reacts with other pollutants, leading to the formation of hazy air (photochemical smog) in urban areas.
- Water Pollution: NO₂ dissolves in water, forming nitric acid, which contaminates water bodies and disrupts aquatic ecosystems.
- Ground-Level Ozone (O₃) Generation: NO₂ plays a crucial role in the production of ground-level ozone, a harmful air pollutant.
- Particulate Matter (PM) Formation: NO₂ contributes to the creation of fine particulate matter (PM₂.₅ and PM₁₀), further degrading air quality.
- Acid Rain: When NO₂ interacts with moisture in the atmosphere, it forms acid rain, which damages infrastructure, soil, and forests.
- Impact on Vegetation: NO₂ disrupts plant growth, reducing chlorophyll content and acting as a stressor to crops and forests.
- NOₓ and Secondary Pollutants: NO₂, along with other nitrogen oxides, reacts with atmospheric chemicals to generate additional pollutants, worsening air quality.
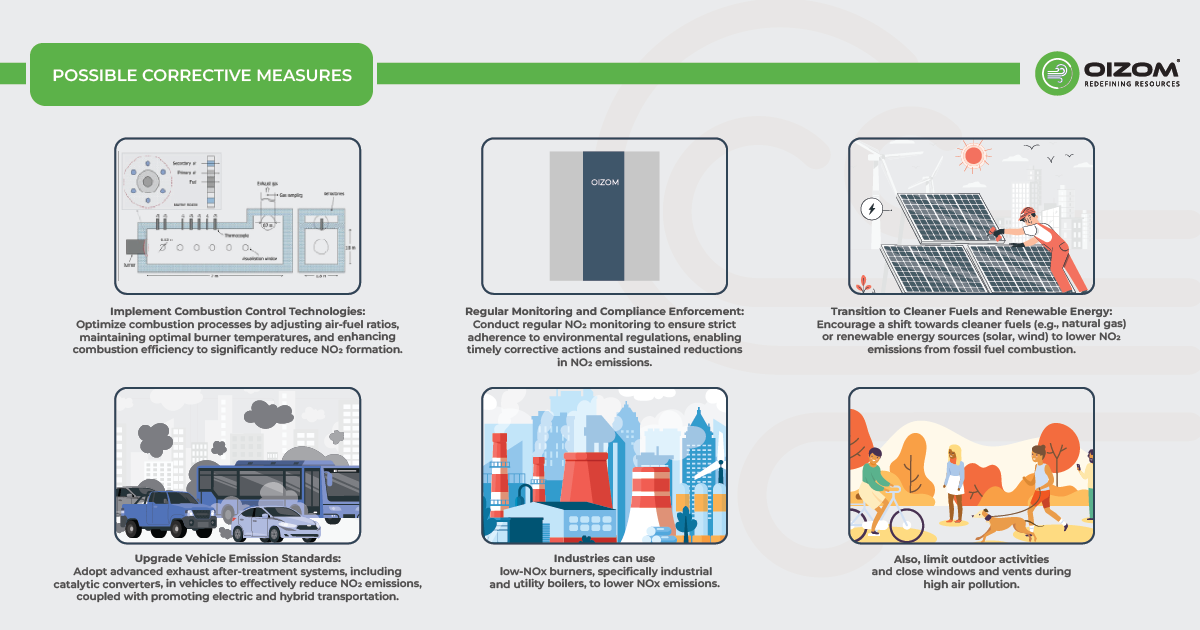
6. Possible Corrective Measures
The major preventive action is to monitor the levels of NO₂, for which our Oizom air quality monitors are very effective. Aside from that, certain preventative methods could help us stay safe from the respiratory effects of this gas:
- Vehicles with low emission rates can help us limit the production of NO₂.
- Alternative fuels, such as hydrogen cells and electric vehicles, can help to reduce NO₂ emissions.
- Avoid traffic congestion. Improving engines’ efficiency. Breathing such polluted air daily also leads to health effects in the long run.
- Have a walk, use bicycles, public transport, or carpool whenever possible.
- Breathing such polluted air daily also leads to health effects in the long run.
- Also, limit outdoor activities and close windows and vents during high air pollution.
- Industries can use low-NOx burners, specifically industrial and utility boilers, to lower NOx emissions.
- A confined environment is used to create fertilizer and process the gasses produced within it.
- A study found that the Lauraceae family plant Glutinosa species reduces NOx in the atmosphere and can be planted near sources of NOx emissions (Highways and roadways).
7. Understanding Nitrogen Dioxide Health Advisory Levels
| Levels | (µg/m3) | Health Effects |
|---|---|---|
| Good | 0-40 | Fresh Air |
| Satisfactory | 41-80 | Coughing, difficulty in breathing experienced |
| Moderately Polluted | 81-180 | Breathing difficulties, aggravation of asthma |
| Poor | 181-280 | Reduced brain functionality and lung function alterations |
| Very poor | 281-400 | Brain damage and heart failure |
| Severe | 400+ | Life-threatening |
Source -NAQI as per CBCB. 2-h hourly average values
8. Measurement Methods of NO2 Monitoring
There are several well-established monitoring methods for NO2, some of which can only measure NO2, while others can also measure NO and/or NOx. Chemiluminescence, cavity-attenuated phase shift spectroscopy (CAPS), semiconductors, and electrochemistry are different working principles for nitrogen oxide monitoring in the ambient environment.
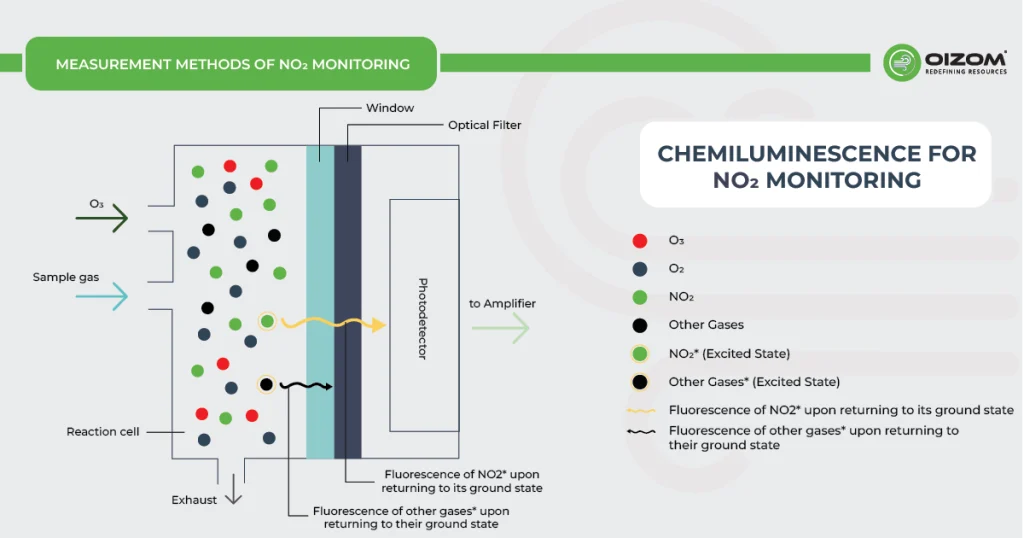
Chemiluminescence for NOx monitoring is the most commonly used conventional method of measuring the NOx levels in the air. It is based on a chemiluminescent reaction between nitric oxide (NO) and ozone (O3). In the NOx monitor, the atmosphere is drawn into two paths. In the first path, the NO2 in the sample is quantitatively reduced to NO using a converter. This converted NO2 and NO in the air reacts with ozone (produced utilizing an ozone generator) to generate activated NO2. During the conversion, light energy at a specific wavelength is proportional to the amount of NO+NO2 in the air sample. In the second path, the air directly reacts with O3, i.e., without passing through the reactor, and the light energy produced is directly proportional to the NO present in the air. The light intensity is measured and recorded photometrically from both paths. The signal from the second path is the amount of NO present in the air sample, while the electronic difference from both paths is the amount of NO2 present in the air sample.
This principle is used for continuous concentration measurement of nitrogen oxides (NOx: NO + NO2), NO, NO2, and ammonia (NH3) in sample gases.
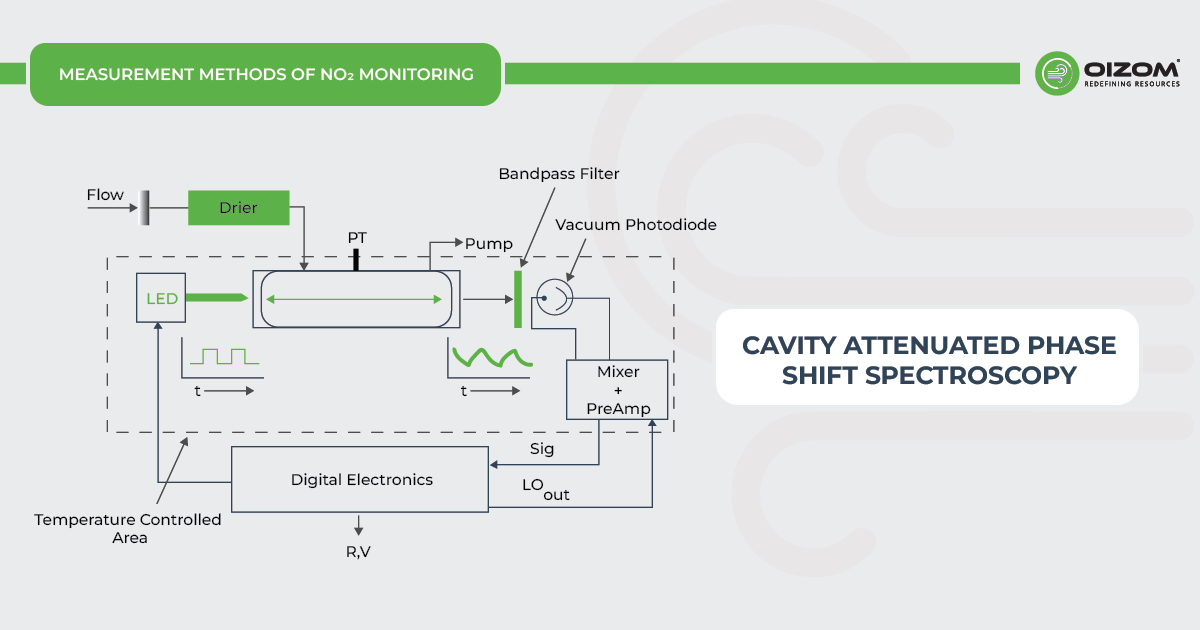
Cavity Attenuated Phase Shift Spectroscopy (CAPS) for NO2 Monitoring – The NO2 monitor working with the CAPS principle operates as an optical absorption spectrometer that measures NO2 directly. Measurement is carried out in a temperature-controlled optical cell containing highly reflective mirrors on both sides to provide extensive optical path length, a blue UV light-emitting diode (LED) centered at 450 nm, and a vacuum photodiode detector. The NO2 present in the air drawn into the NO2 monitor causes a phase shift in the signal received by a photodetector proportional to the NO2 concentration.
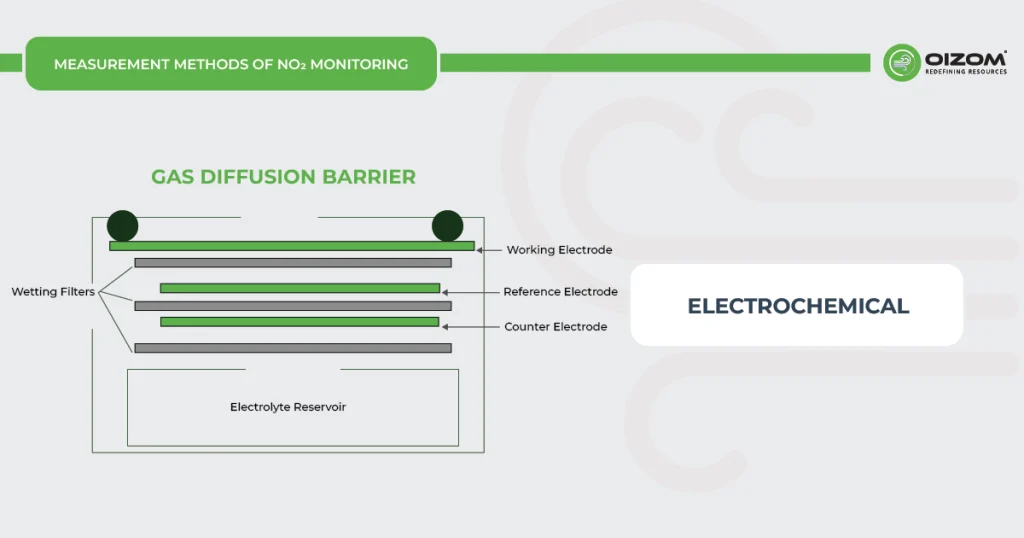
Electrochemical method for NOx Monitoring – NOx monitors working on the electrochemical principle are operated based on the diffusion of nitrogen dioxide and nitric oxide gas into the respective sensor, producing electrical signals proportional to the NO2 and NO concentration, respectively. It allows accurate measurement of even low concentrations of NOx, which is essential in NO2 and NO monitoring for the ambient air.
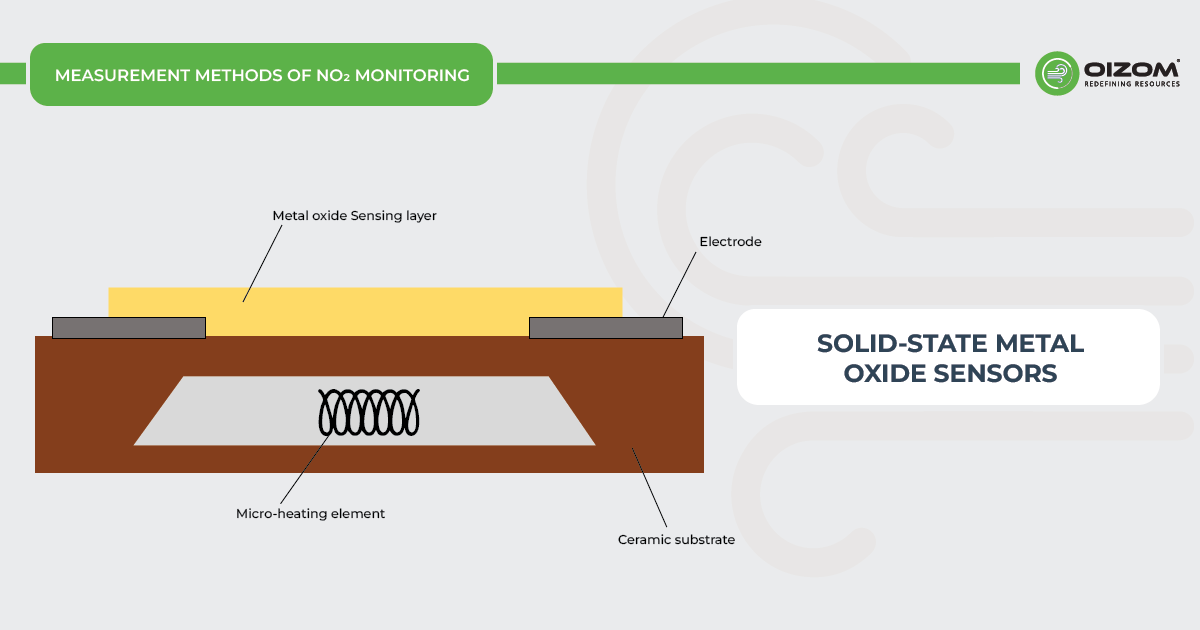
Solid-State Metal Oxide Sensors
Metal Oxide Sensors are a prominent sensor technology for measuring NOx gas concentrations. Metal Oxide Sensors comprise a heating and semiconducting metal oxide sensing elements. The heater heats the sensor element’s surface to 300°-500°C, allowing it to detect gases via a chemical reaction on its surface. This reaction alters the electrical conductivity of the sensing element, which may be measured using an external circuit to determine the observed gas level.
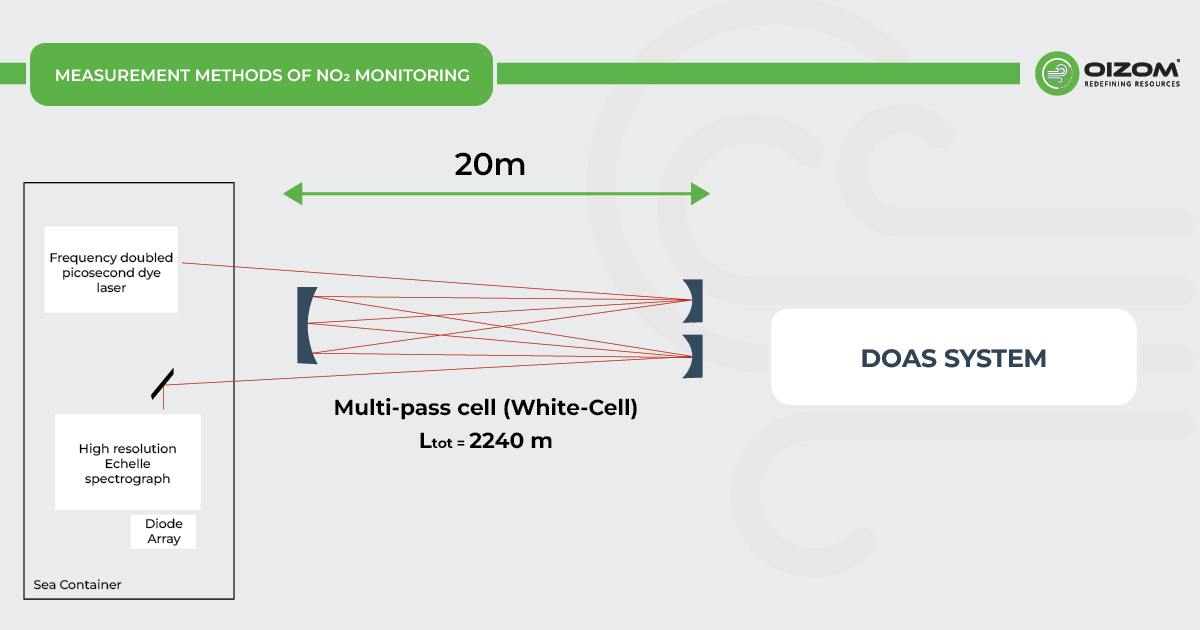
DOAS System (Differential Optical Absorption Spectroscopy)
The Differential Optical Absorption Spectroscopy (DOAS) system operates by measuring the absorption of light at specific wavelengths as it passes through a column of air. It consists of an emitter, receiver, and analyzer. The emitter projects light, including visible, ultraviolet, and infrared wavelengths, across a path of several hundred meters. The amount of light absorbed correlates with the concentration of pollutants, based on Beer-Lambert’s Law.
The receiver captures the transmitted light and directs it to the analyzer. The analyzer uses a spectrometer to split the light into narrow wavelength bands and compares the absorption data with reference spectra. This process allows for the simultaneous detection and quantification of multiple gases by analyzing their unique absorption features.
This system provides high-precision pollutant measurements in real-time, making it effective for monitoring air quality across large distances.
Comparison of Monitoring Techniques
| Method | Accuracy | Cost | Complexity | Suitable Applications |
|---|---|---|---|---|
| Chemiluminescence | Very High | High | High | Regulatory monitoring |
| CAPS | High | Moderate-High | Moderate | Urban air quality studies |
| Electrochemical | Moderate-High | Low | Low | Portable/personal monitors |
| Metal Oxide Sensors | Moderate | Low | Low | IoT-based network monitoring |
| DOAS | High | Very High | High | City-scale pollutant mapping |
9. Oizom’s Sensor Working Principle for NO2 Monitoring
Oizom provides a range of nitrogen dioxide (NO2) and Nitric Oxide (NO) sensor modules to monitor varying NO2 and NO levels based on your needs. Our sensors accurately measure NO2 and NO in ambient conditions, detecting concentrations in ppb/ppm. This sensor monitors nitrogen dioxide and nitric oxide in real-time. This sensor is integrated into a metal casing and ultra-low-noise support electronics, making it compact and reliable. This allows accurate gas detection even at very low concentrations in the atmosphere. This sensor works on the Electrochemical working principle to measure environmental air quality.
This sensor undergoes calibration using standard gases and tools to ensure high sensitivity and accuracy. Each gas sensor is calibrated with zero and span checks in a controlled lab, following Section 12.2 of the USEPA Quality Assurance Handbook for Air Pollution Measurement Systems Volume II. Calibration is done using NIST-traceable gas standards for reliable performance.
The NO2 and NO sensor module is integrated into outdoor air quality monitoring systems like Polludrone, AQBot, and Odosense. It is ideal for campus monitoring, smart cities, industries, wastewater treatment plants, roads, research projects, and environmental impact assessments. By utilizing this sensor module, users can ensure they receive accurate, real-time data on NO2 and NO levels, aiding in the effective monitoring and management of air quality across diverse environments.
10. Why Choose Oizom NO2 and NO Sensor?
- space, making them ideal for portable air quality monitoring. The NO2 and NO sensors come pre-calibrated and can be quickly replaced in just a few minutes by removing and replacing the old sensor with a new one.
- Durable: The NO2 and NO sensors have a long life of almost two years.
- Energy Efficient: There is no need to be concerned about energy usage because our sensors are not only accurate but also energy efficient. Powered up with just 3.3 to 5V for efficient, reliable performance!
- In-house sensor tech: Oizom applies advanced data processing algorithms to compensate for the effects of temperature and humidity on the sensor output. The algorithm is designed to automatically update based on weather conditions and seasonal changes, removing its influence on the sensor performance. The advanced algorithms also compensate for the effect of the cross-sensitive gas.
- Ultra-Low Noise Electronics: The sensor module’s design is crucial for accurate measurements. Each sensor is housed in a metal casing with ultra-low-noise electronics and a base PCB. The metal casing shields the sensor and electronics from electromagnetic interference, preventing false readings.
- RoHS Compliant: Our sensors comply with the RoHS criteria for restricting hazardous substances in electrical and electronic devices.
11. 5 Reasons Why NO2 and NO Monitoring is Important
- NO2 and NO are major pollutants from vehicular emissions and energy production, resulting in poor air quality in almost all urban regions.
- NOx, apart from affecting human health and the environment itself, has the potential to produce “secondary pollutants” such as ozone, smog, nitric acid, and particulate matter, which have adverse effects on health and the environment.
- When inhaled, NOx causes damage to lung tissue and a reduction in the functioning of the respiratory system, making children, people with lung diseases such as asthma, and people who work or exercise outside more susceptible to adverse health impacts.
- NOx monitoring is an efficient way to prevent the accumulation of high levels of NOx. It helps detect the amount of NOX we breathe in and alerts us when a certain level is exceeded.
Real-time monitoring of NOx levels helps calculate the air quality index, which is used to issue health advisories and formulate an action plan to meet standards.
FAQs
NO₂ is a toxic air pollutant from combustion processes like vehicle emissions and industrial activities. It can cause respiratory issues heart problems, and contribute to smog and acid rain.
The primary sources of NO₂ are vehicle exhaust, power plants, industrial processes, and biomass burning. Indoor sources include gas stoves and kerosene heaters.
Exposure to NO₂ can lead to breathing difficulties, asthma attacks, lung inflammation, reduced immunity, and an increased risk of heart disease and premature death.
NO₂ contributes to smog, acid rain, increased particulate matter (PM₂.₅), and ozone (O₃) formation. It also harms vegetation by reducing chlorophyll content and stunting plant growth.
Using low-emission vehicles, adopting alternative fuels like hydrogen and electric, improving engine efficiency, using public transport, and controlling industrial emissions can help lower NO₂ levels.