Did you know? Nitrogen (N) is a fundamental element that makes up about 78% of the Earth’s atmosphere. When two nitrogen atoms bond, they form nitrogen gas (N₂), a stable, inert gas essential to life on Earth. However, under high-temperature conditions, like those occurring during fossil fuel combustion, nitrogen combines with oxygen to form nitrogen oxides (NOx). The most common nitrogen oxides are nitric oxide (NO) and nitrogen dioxide (NO₂). Nitrogen dioxide, in particular, is a significant air pollutant known to harm human health and the environment.
Nitric oxide (NO) naturally occurs through lightning strikes and soil microbial processes. Yet, its major contributors are combustion processes, such as vehicle engines, industrial furnaces, and power plants, where nitrogen from the air reacts with oxygen under extreme heat. Although NO plays important biological roles inside the human body, it is not directly emitted by human respiration. NO doesn’t affect us in its natural form.
This article thoroughly explores nitric oxides, covering their primary sources, permissible ambient air concentrations, impacts on human health and ecosystems, corrective measures for reducing emissions, the importance of monitoring NO levels, and an overview of effective NO monitoring methods. Understanding nitrogen oxides helps us manage and improve air quality, protecting both our health and the environment.
1. What is Nitric Oxide (NO)?
Nitric Oxide (NO), also known as Nitrogen Monoxide, is a colorless, highly reactive gaseous pollutant with a distinctive sweet odor. Chemically, it consists of one nitrogen atom bonded to one oxygen atom. The molecule possesses a single unpaired electron, making it extremely reactive and prone to rapid oxidation in the atmosphere. Under ambient conditions, NO typically converts to nitrogen dioxide (NO₂) within a few minutes.
NO is classified as a primary air pollutant, primarily emitted directly from combustion sources. Its monitoring is critical due to its role as a precursor to harmful secondary pollutants such as nitrogen dioxide (NO₂), ozone (O₃), and nitric acid (HNO₃).
2. What’s the difference between NOX, NO and NO2?
- The phrase “nitrogen oxides” (NOx) refers to both nitrogen dioxide (NO2) and nitrogen monoxide (NO).
- Nitrogen monoxide (NO) is a colorless gas and one of the primary oxides of nitrogen.
- Nitrogen dioxide (NO2) is a reddish-brown gas with a unique, acidic odor. It is one of several nitrogen oxides.
Nitric Oxide: Nitrogen monoxide (NO), often known as nitric oxide, is a colorless gas with a strong, sweet odor and a hazardous air pollutant. It consists of one nitrogen atom bonded to one oxygen atom. Due to one unpaired electron, it is highly reactive and thus rapidly oxidized (within a few minutes) to NO2.
Nitrogen Dioxide: Nitrogen dioxide (NO2) is a critical air pollutant highlighted by air quality guidelines like those from the World Health Organization. NO2 is a reddish-brown acidic gas with a pungent, dissolves in water, and acts as a strong oxidant and corrosive. It forms when nitric oxide (NO) oxidizes during combustion, such as in diesel engines and power plants burning coal, oil, gas, wood, or waste. NO2 gas itself is not flammable. However, it can accelerate the burning of combustible materials.
Nitric oxide (NO) in the Atmosphere
It is a colorless, highly reactive gas naturally present in Earth’s atmosphere. Although naturally formed through lightning strikes and soil microbial processes, its main atmospheric source is combustion, especially from vehicles, power generation, and industrial processes. Due to its unpaired electron, nitric oxide quickly oxidizes, typically within minutes, to form nitrogen dioxide (NO₂), a harmful secondary pollutant. This rapid transformation significantly contributes to urban air pollution, smog formation, and atmospheric chemistry dynamics, including the formation of tropospheric ozone.
Atmospheric NO also indirectly affects ecosystems by leading to acid rain, soil acidification, and nutrient imbalance, negatively impacting biodiversity and vegetation health. Monitoring nitric oxide in the atmosphere is therefore critical, not only to assess immediate air quality but also to predict and manage the formation of secondary pollutants. Effective NO measurement and management help mitigate its environmental and health impacts, promoting healthier ecosystems and improved public health.
3.Sources of Nitric Oxide (NO)
Nitric Oxide predominantly arises from combustion processes involving high temperatures, but natural sources also contribute significantly:
Anthropogenic (Human-made) Sources:
- Vehicular Emissions: Internal combustion engines, particularly gasoline and diesel vehicles, emit large quantities of NO due to high-temperature reactions between atmospheric nitrogen and oxygen.
- Industrial Combustion: Power plants, cement kilns, waste incinerators, and industrial boilers generate substantial NO emissions through combustion processes, primarily via thermal oxidation (Zeldovich mechanism).
- Fuel Combustion: Combustion of fuels containing organic nitrogen (e.g., coal and biomass) leads to the direct emission of NO through fuel-NO formation.
Natural Sources:
- Lightning Strikes: Natural formation of nitrogen oxides, including NO, occurs notably during lightning events. A single lightning strike, reaching temperatures up to 30,000 kelvins (~53,540 °F), facilitates reactions converting atmospheric nitrogen and oxygen directly into NO. Lightning strikes Earth approximately 100 times per second globally, and these events significantly contribute to the ambient NO levels.
- Biogenic Processes: Soil microbial activity (nitrification and denitrification) and wildfires produce minor but measurable amounts of NO.
4. Permissible levels of NO
Globally, regulatory standards typically address nitrogen oxides collectively as NOx (including NO and NO₂). Specifically, for nitric oxide (NO), standards are often implied within broader NOx limits rather than established separately, as NO quickly converts to NO₂ in the atmosphere.
Below are the breakpoint concentrations describing air quality based on nitrogen dioxide concentrations for different countries. In India, daily NO2 levels of up to 80 µg/m3 are considered satisfactory.
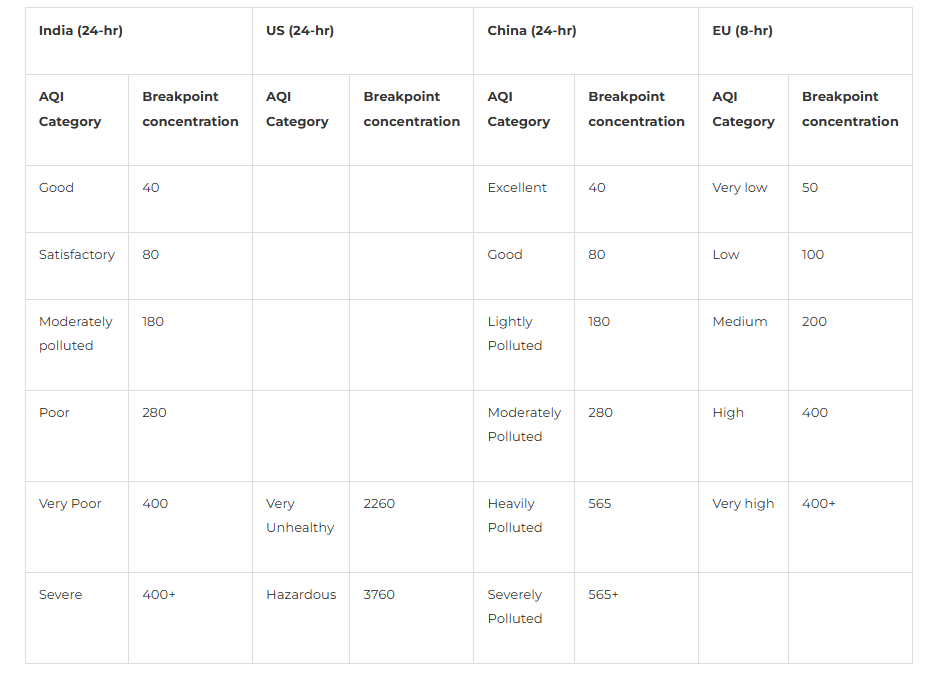
[Source: National Ambient Air Quality Index, CPCB (Oct 2014)]
The World Health Organization (WHO) has updated its air quality guidelines to better protect public health. The new NO2 guideline is set at 10 µg/m³ as an annual average, marking a significant reduction of 30 µg/m³ from the previous guideline established in 2005. This update underscores the WHO’s commitment to enhancing global air quality standards to safeguard public health.
The table below will represent WHO Indoor and Outdoor Air Quality Guidelines for the NO2
| Source | Indoor Air Quality | Outdoor Air Quality |
|---|---|---|
| NO2 | 1 Hour = 200 µg/m3 Annual = 40 µg/m3 | 24 Hour = 25 µg/m3 Annual = 10 µg/m3 |
Source – National Library of Medicine
WORKPLACE EXPOSURE LIMITS for NO2
- OSHA: The legal airborne permissible exposure limit (PEL) is 5 ppm, not to be exceeded at any time.
- NIOSH: The recommended airborne exposure limit is 1 ppm, which should not be exceeded at any time.
- ACGIH: The recommended airborne exposure limit is 3 ppm averaged over an 8-hour workshift, and 5 ppm as a STEL (short-term exposure limit).
*Nitrogen Dioxide may cause mutations. All contact with this chemical should be reduced to the lowest possible level
5. Health & Environmental Impact of NO
While nitric oxide itself is not considered directly harmful at typical ambient concentrations, its rapid atmospheric oxidation to nitrogen dioxide (NO₂) has serious implications for both human health and environmental ecosystems:
Human Health Impacts:
- Respiratory Concerns: Although NO alone has minimal direct respiratory effects, it quickly transforms into NO₂, which can irritate the respiratory tract, exacerbate asthma, and reduce lung function.
- Secondary Pollutants: NO is instrumental in the photochemical formation of ground-level ozone (O₃), a respiratory irritant linked to chronic respiratory diseases, cardiovascular problems, and adverse health outcomes.
Environmental Impacts:
- Formation of Acid Rain: NO oxidation products, primarily nitric acid (HNO₃), contribute to acid deposition, leading to acidification of soils, freshwater ecosystems, and forests. This acidification disrupts nutrient cycling, diminishes biodiversity, and negatively affects ecosystem productivity.
- Ecosystem and Biodiversity Loss: Prolonged exposure to nitrogen oxides alters terrestrial and aquatic ecosystems, facilitating eutrophication, reduced biodiversity, and habitat degradation.
- Tropospheric Ozone Formation: The conversion of NO to NO₂ catalyzes reactions that produce tropospheric ozone, harming vegetation, reducing crop yields, and contributing to global climate change.
Given these impacts, accurate and continuous monitoring of NO concentrations is essential to inform effective pollution-control measures, regulatory compliance, and proactive environmental management.
6. Possible corrective measures
The major preventive action is to monitor the levels of NO, for which our Oizom air quality monitors are very effective. Aside from that, certain preventative methods could help us stay safe from the respiratory effects of this gas:
Implement Combustion Control Technologies:
- Optimize combustion processes by adjusting air-fuel ratios, maintaining optimal burner temperatures, and enhancing combustion efficiency to significantly reduce NO formation.
Use of NOx Reduction Systems (SCR and SNCR):
- Deploy Selective Catalytic Reduction (SCR) and Selective Non-Catalytic Reduction (SNCR) technologies in industrial boilers and power plants to chemically reduce emitted NO into harmless nitrogen (N₂).
Transition to Cleaner Fuels and Renewable Energy:
- Encourage a shift towards cleaner fuels (e.g., natural gas) or renewable energy sources (solar, wind) to lower NO emissions from fossil fuel combustion.
Upgrade Vehicle Emission Standards:
- Adopt advanced exhaust after-treatment systems, including catalytic converters, in vehicles to effectively reduce NO emissions, coupled with promoting electric and hybrid transportation.
Regular Monitoring and Compliance Enforcement:
- Conduct regular NO monitoring to ensure strict adherence to environmental regulations, enabling timely corrective actions and sustained reductions in NO emissions.
7. Measurement methods of NO monitoring
There are several well-established monitoring methods for NO2, some of which can only measure NO2, while others can also measure NO and/or NOx. Chemiluminescence, cavity-attenuated phase shift spectroscopy (CAPS), semiconductors, and electrochemistry are different working principles for nitrogen oxide monitoring in the ambient environment.
Chemiluminescence for NOx monitoring is the most commonly used conventional method of measuring the NOx levels in the air. It is based on a chemiluminescent reaction between nitric oxide (NO) and ozone (O3). In the NOx monitor, the atmosphere is drawn into two paths. In the first path, the NO2 in the sample is quantitatively reduced to NO using a converter. This converted NO2 and NO in the air reacts with ozone (produced utilizing an ozone generator) to generate activated NO2. During the conversion, light energy at a specific wavelength is proportional to the amount of NO+NO2 in the air sample. In the second path, the air directly reacts with O3, i.e., without passing through the reactor, and the light energy produced is directly proportional to the NO present in the air. The light intensity is measured and recorded photometrically from both paths. The signal from the second path is the amount of NO present in the air sample, while the electronic difference from both paths is the amount of NO2 present in the air sample.
This principle is used for continuous concentration measurement of nitrogen oxides (NOx: NO + NO2), NO, NO2, and ammonia (NH3) in sample gases.
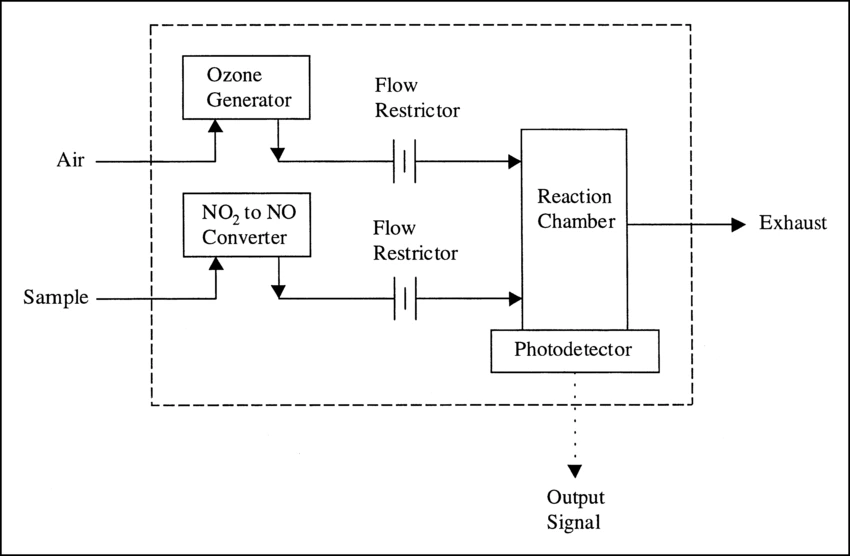
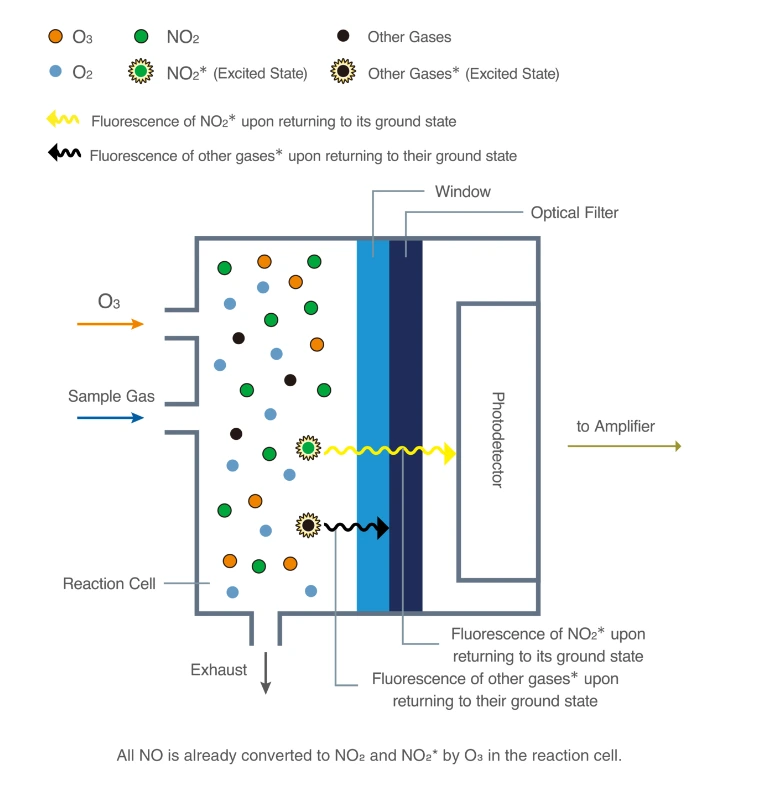
Figure – Structure and operating principle of a gas analyzer using the chemiluminescence method.
Source- https://www.horiba.com/int/process-and-environmental/measuring-principles/chemiluminescence/
Cavity Attenuated Phase Shift Spectroscopy (CAPS) for NO2 monitoring – The NO2 monitor working with the CAPS principle operates as an optical absorption spectrometer that measures NO2 directly. Measurement is carried out in a temperature-controlled optical cell containing highly reflective mirrors on both sides to provide extensive optical path length, a blue UV light-emitting diode (LED) centered at 450 nm, and a vacuum photodiode detector. The NO2 present in the air drawn into the NO2 monitor causes a phase shift in the signal received by a photodetector proportional to the NO2 concentration.
Electrochemical method for NOx monitoring – NOx monitors working on the electrochemical principle are operated based on the diffusion of nitrogen dioxide and nitric oxide gas into the respective sensor, producing electrical signals proportional to the NO2 and NO concentration, respectively. It allows accurate measurement of even low concentrations of NOx, which is essential in NO2 and NO monitoring for the ambient air.
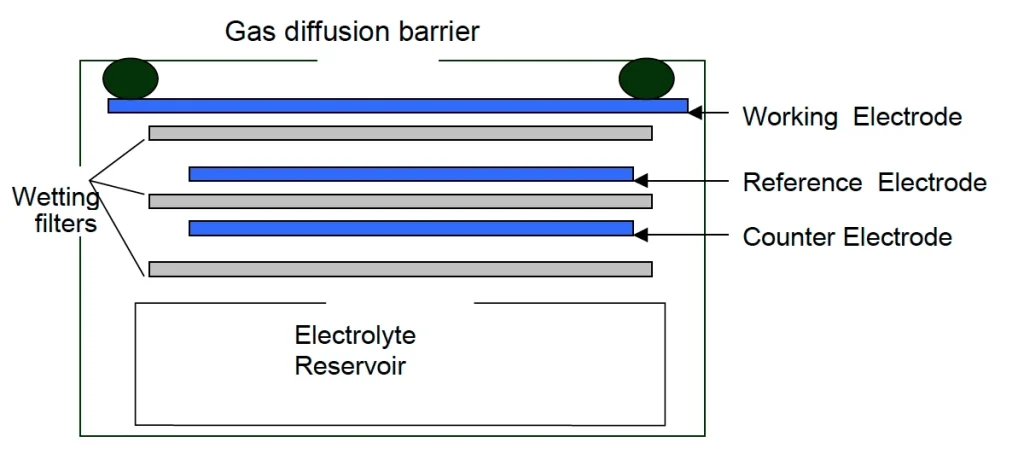
[Source: https://docs.smartcitizen.me/Components/sensors/Electrochemical%20Sensors/]
Solid-State Metal Oxide Sensors
Metal Oxide Sensors are a prominent sensor technology for measuring NOx gas concentrations. Metal Oxide Sensors comprise a heating and semiconducting metal oxide sensing elements. The heater heats the sensor element’s surface to 300°-500°C, allowing it to detect gases via a chemical reaction on its surface. This reaction alters the electrical conductivity of the sensing element, which may be measured using an external circuit to determine the observed gas level.
DOAS System (Differential Optical Absorption Spectroscopy)
The Differential Optical Absorption Spectroscopy (DOAS) system operates by measuring the absorption of light at specific wavelengths as it passes through a column of air. It consists of an emitter, receiver, and analyzer. The emitter projects light, including visible, ultraviolet, and infrared wavelengths, across a path of several hundred meters. The amount of light absorbed correlates with the concentration of pollutants, based on Beer-Lambert’s Law.
The receiver captures the transmitted light and directs it to the analyzer. The analyzer uses a spectrometer to split the light into narrow wavelength bands and compares the absorption data with reference spectra. This process allows for the simultaneous detection and quantification of multiple gases by analyzing their unique absorption features.
This system provides high-precision pollutant measurements in real-time, making it effective for monitoring air quality across large distances.
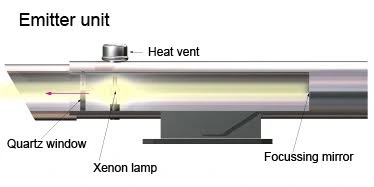
Fig – Illustration of a Differential Optical Absorption Spectroscopy emitter unit
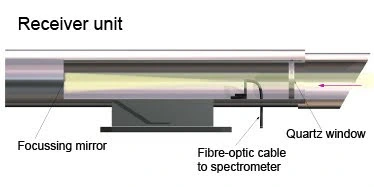
Fig – Illustration of a Differential Optical Absorption Spectroscopy Receiver unit
Comparison of Monitoring Techniques
| Method | Accuracy | Cost | Complexity | Suitable Applications |
|---|---|---|---|---|
| Chemiluminescence | Very High | High | High | Regulatory monitoring |
| CAPS | High | Moderate-High | Moderate | Urban air quality studies |
| Electrochemical | Moderate-High | Low | Low | Portable/personal monitors |
| Metal Oxide Sensors | Moderate | Low | Low | IoT-based network monitoring |
| DOAS | High | Very High | High | City-scale pollutant mapping |
8. Oizom’s sensor working principle for NO monitoring
Oizom provides a range of nitrogen dioxide (NO2) and Nitric Oxide (NO) sensor modules to monitor varying NO2 and NO levels based on your needs. Our sensors accurately measure NO2 and NO in ambient conditions, detecting concentrations in ppb/ppm. This sensor monitors nitrogen dioxide and nitric oxide in real-time. This sensor is integrated into a metal casing and ultra-low-noise support electronics, making it compact and reliable. This allows accurate gas detection even at very low concentrations in the atmosphere. This sensor works on the Electrochemical working principle to measure environmental air quality.
This sensor undergoes calibration using standard gases and tools to ensure high sensitivity and accuracy. Each gas sensor is calibrated with zero and span checks in a controlled lab, following Section 12.2 of the USEPA Quality Assurance Handbook for Air Pollution Measurement Systems Volume II. Calibration is done using NIST-traceable gas standards for reliable performance.
The NO2 and NO sensor module is integrated into outdoor air quality monitoring systems like Polludrone, Pollusense, AQBot, and Odosense. It is ideal for campus monitoring, smart cities, industries, wastewater treatment plants, roads, research projects, and environmental impact assessments. By utilizing this sensor module, users can ensure they receive accurate, real-time data on NO2 and NO levels, aiding in the effective monitoring and management of air quality across diverse environments.
9. Why Choose Oizom NO2 and NO Sensor?
- Compact: Our sensors are small and easy to install, perfect for use in any space, making them ideal for portable air quality monitoring. The NO2 and NO sensors come pre-calibrated and can be quickly replaced in just a few minutes by removing and replacing the old sensor with a new one.
- Durable: The NO2 and NO sensors have a long life of almost two years.
- Energy Efficient: There is no need to be concerned about energy usage because our sensors are not only accurate but also energy efficient. Powered up with just 3.3 to 5V for efficient, reliable performance!
- In-house sensor tech: Oizom applies advanced data processing algorithms to compensate for the effects of temperature and humidity on the sensor output. The algorithm is designed to automatically update based on weather conditions and seasonal changes, removing its influence on the sensor performance. The advanced algorithms also compensate for the effect of the cross-sensitive gas.
- Ultra-Low Noise Electronics: The sensor module’s design is crucial for accurate measurements. Each sensor is housed in a metal casing with ultra-low-noise electronics and a base PCB. The metal casing shields the sensor and electronics from electromagnetic interference, preventing false readings.
- RoHS Compliant: Our sensors comply with the RoHS criteria for restricting hazardous substances in electrical and electronic devices.
10. 5 Reasons why NO2 and NO monitoring is important
- NO2 and NO are major pollutants from vehicular emissions and energy production, resulting in poor air quality in almost all urban regions.
- NOx, apart from affecting human health and the environment itself, has the potential to produce “secondary pollutants” such as ozone, smog, nitric acid, and particulate matter, which have adverse effects on health and the environment.
- When inhaled, NOx causes damage to lung tissue and a reduction in the functioning of the respiratory system, making children, people with lung diseases such as asthma, and people who work or exercise outside more susceptible to adverse health impacts.
- NOx monitoring is an efficient way to prevent the accumulation of high levels of NOx. It helps detect the amount of NOX we breathe in and alerts us when a certain level is exceeded.
- Real-time monitoring of NOx levels helps calculate the air quality index, which is used to issue health advisories and formulate an action plan to meet standards.
FAQs
Nitric Oxide (NO) is a colorless, reactive gas rapidly converting to Nitrogen Dioxide (NO₂), a reddish-brown, toxic pollutant affecting respiratory health and contributing significantly to smog and acid rain.
Continuous NO monitoring helps precisely track emission sources and dynamics of pollutant transformation, which is crucial for timely air quality interventions and regulatory compliance.
Chemiluminescence analyzers provide the highest accuracy and reliability, making them the preferred method for regulatory compliance and official air-quality assessments.
Yes, temperature and humidity significantly influence NO sensor readings, necessitating advanced calibration and algorithms (used by Oizom sensors) to compensate effectively.
Industries can adopt advanced combustion controls, install selective catalytic/non-catalytic reduction technologies, transition to cleaner fuels, and maintain rigorous emission monitoring and compliance standards.