Key Takeaway Points
- The greenhouse effect, explored by researchers like Eunice Foote and Svante Arrhenius, involves warming the Earth’s surface due to specific gases known as greenhouse gases.
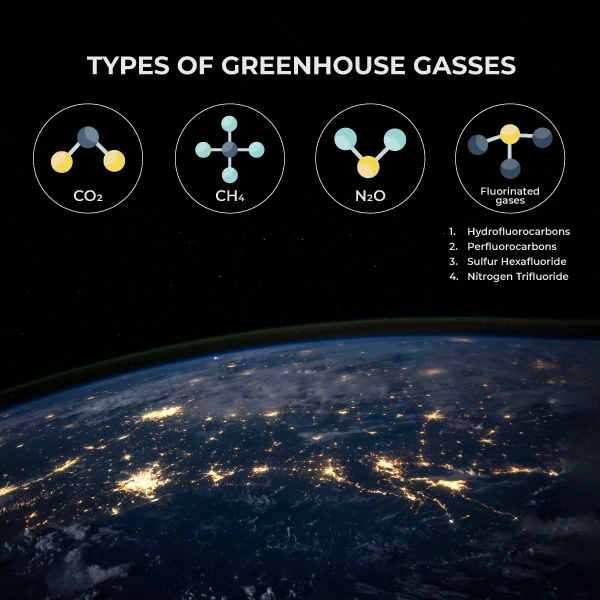
- The six major greenhouse gases are carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and fluorinated gases (F-gases).
- CO2, the most prevalent greenhouse gas, has doubled since the Industrial Revolution, primarily from burning fossil fuels and industrial processes. Though less prevalent, methane is more potent in trapping heat and is emitted from natural gas production and agricultural practices.
- N2O levels have increased due to agricultural and industrial activities, and it is 300 times more effective at trapping heat than CO2.
- F-gases, entirely man-made, are used in refrigeration and electronics and are highly potent and long-lasting.
- Greenhouse gases trap heat, causing global warming.
- Solutions like Polludrone from Oizom can help monitor emissions in real time and provide accurate data. Reducing emissions requires global cooperation and region-specific policies to combat climate change.
- Understanding the different types of greenhouse gases and their impact is crucial for making informed decisions and combating climate change.
- Spreading awareness about the dangers of greenhouse gases is essential for protecting our health and the environment.
The Role of Different Greenhouse Gases: Understanding it in Details

Long before global warming became a hot topic, people used to talk about the “greenhouse effect” to reflect on their impact on the climate. We don’t know exactly when this phrase first appeared, but the idea was already in scientific works by the late 19th century. Researchers like Eunice Foote, Svante Arrhenius, and John Tyndall were all exploring the concept back then. You might be surprised or wondering who discovered the phenomenon of the greenhouse effect. Then, here you are at the right place to learn about greenhouse gasses. The above-highlighted sentence indicates the researchers who discovered the greenhouse effect.
Nowadays, the term “greenhouse effect” is used frequently. But are we truly sure what it means? How is it harmful? How is the world dealing with a problem as significant as this? The greenhouse effect is described as warming the Earth’s surface and troposphere (the lowest layer of the atmosphere) caused by specific gasses in the air. These gasses are referred to as greenhouse gases. In this blog, I will discuss the various types of greenhouse gases.
Types of greenhouse gasses
There are six major types of greenhouse gases, each having unique features. Carbon dioxide is the most released greenhouse gas on Earth (about 76%). Hence, decarbonization is the most commonly discussed strategy for combating climate change. It is followed by fluorinated gases (2%), nitrous oxide (6%), and methane (16%).
Carbon dioxide (CO2)
- The most well-known greenhouse gas is carbon dioxide. This chemical molecule has one carbon atom linked to two oxygen atoms, giving the formula CO2. Before the Industrial Revolution, CO2 was a trace gas in our atmosphere, averaging around 228 parts per million. However, its current level has nearly doubled, reaching 421 parts per million. This growth is to blame for rising global temperatures and climate change.
- (CO2) is a colorless gas with a faint, severe odour and a sour taste. It is one of the most significant greenhouse gases associated with global warming. It is produced during carbon-containing material combustion, fermentation, and animal respiration and is used by plants in the photosynthesis of carbohydrates.
- Carbon dioxide enters the atmosphere through the combustion of fossil fuels (coal, natural gas, and oil), solid waste, trees, and other biological materials, as well as chemical processes (for example, cement manufacturing).
Sources
Many living species in our world, including plants, animals, soils, oceans, and volcanoes, naturally emit carbon dioxide as they breathe and decay. However, we should be concerned about the CO2 emitted by humans, which accounts for around 75% of human-caused emissions. The vast majority is derived from using fossil fuels such as coal, oil, and gas for electricity and transportation, as well as forestry and land management.
Monitoring CO2 and other gases with polludrone can be very helpful for industries in getting accurate real-time data. Polludrone packs a punch with features like integrated sensors enclosed in a single, compact, corrosion and vandalism-proof case, offering unmatched reliability, including in-built calibration software and web-based software.
Impact on Climate Change
Carbon dioxide and other greenhouse gases operate as a blanket or cap, keeping part of the heat that Earth would otherwise release into space. That’s the simplest answer. But how exactly do some molecules capture heat? The solution there involves a dive into physics and chemistry.
Carbon dioxide is Earth’s most important greenhouse gas, absorbing and radiating heat. Unlike oxygen and nitrogen, which comprise most of our atmosphere, greenhouse gases absorb heat radiation from the Earth’s surface and re-release it in all directions, including back toward the Earth’s surface. Without carbon dioxide, Earth’s natural greenhouse effect would be insufficient to keep the average global surface temperature above freezing. People are accelerating the natural greenhouse effect by increasing the amount of carbon dioxide in the atmosphere, causing global temperatures to rise.
Human activity, particularly the combustion of fossil fuels, is clearly responsible for the increase in CO2. Because fossil fuels are millions of years old, their carbon differs chemically from that created by modern natural processes like decomposition or respiration. By examining atmospheric carbon, scientists can directly assess the distinctive chemical signature of fossil fuels, allowing them to comprehend better their role in increasing CO2.
Methane (CH4)
Methane, composed of one carbon atom bound to four hydrogen atoms (CH4), is the environment’s second most harmful greenhouse gas. While atmospheric methane is significantly less prevalent than CO2 (about 1.7 parts per million), its concentration has increased by approximately 150% since pre-industrial times. Furthermore, methane has a far higher heat-trapping power than other gases and is thought to be responsible for around half of the rise in global temperature. Its atmospheric concentrations are usually measured in parts per billion (ppb) rather than parts per million (ppm).
Sources
Methane is released while producing and transporting coal, natural gas, and oil. Methane emissions are also caused by livestock and other agricultural practices, land use, and the breakdown of organic waste in municipal solid waste landfills.
Methane is produced by the breakdown of organic matter in oxygen-poor environments such as marshes, rice paddies, landfills, cattle digestive tracts, and the combustion of fossil fuels. It is believed that 40% of global methane emissions come from natural sources, while 60% come from human activities, namely energy (fossil fuels), agriculture, and garbage.
Impact on Climate Change
Methane is absorbed primarily in the troposphere (the lowest layer of the atmosphere), where it interacts with other chemicals to produce water and CO2. However, forest soils play an essential function as methane sinks because microbes break it down into smaller components that can be used for energy. Unfortunately, pollution and deforestation have reduced soil methane absorption by 77% during the last 30 years.
This gas is particularly usable for generating energy and most methane reduction technologies, including landfill gas recovery from waste or biogas produced from agricultural manure.
Nitrous oxide (N2O)
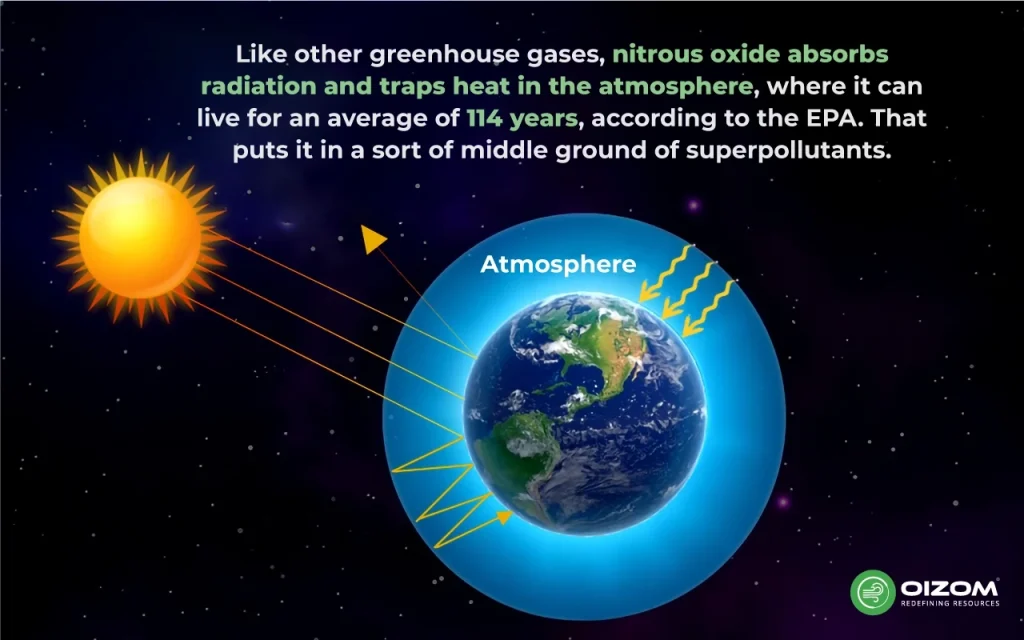
Nitrous oxide (N2O), also known as laughing gas, is a nonflammable gas containing an oxide of nitrogen. While levels of N2O have rarely exceeded 280 parts per billion throughout history, human activity in the previous century dramatically increased them, reaching 334 parts per billion in 2021. This is particularly concerning because N2O is 300 times more effective than carbon dioxide at heating the environment. It is also long-lived, with an average lifetime of 114 years in the atmosphere before disintegration.
Nitrous oxide also poses a second threat: when in the stratosphere, it is exposed to sunlight and oxygen, converting the gas to nitrogen oxides. Nitrogen oxides can deplete the ozone layer, which humans rely on to keep most of the sun’s UV radiation from reaching the earth’s surface.
Sources
Nitrous oxide is produced during agricultural, land use, and industrial operations, the combustion of fossil fuels and solid waste, and wastewater treatment.
- Transportation
- Power generation
- Soil cultivation activities, particularly the use of commercial and organic fertilizers
- Fossil fuel combustion
- Nitric acid generation
- Biomass burning
Impact on Climate Change
Nitrous oxide is 300 times more powerful than carbon dioxide and depletes the ozone layer. Because it also has a shorter life cycle, lowering it may have a faster and more dramatic influence on global warming.
Fluorinated gases
While CO2 and methane occur naturally on our planet, fluorinated gases (F-gases) are entirely man-made. Hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), sulfur hexafluoride (SF6), and nitrogen trifluoride (NF3) were developed in the 1990s as alternatives to ozone-damaging chemicals.
F-gases, which are used in various industrial activities such as refrigeration, electronics, cosmetics, and solvents, are potent and long-lasting greenhouse gases that contribute significantly to global warming.
Fluorinated gases are responsible for 0.34 watts per square meter of radiative forcing. Nitrous oxide is responsible for 0.16 watts per square meter. Nitrous oxides have low background concentrations because of natural biological interactions in soil and water. Whereas fluorinated gases are virtually completely derived from industrial sources.
Sources
Hydrofluorocarbons, perfluorocarbons, sulfur hexafluoride, and nitrogen trifluoride are potent greenhouse gases produced by various domestic, commercial, and industrial operations.
F-gases are emitted by companies that manufacture them and those who use them in their processes or equipment. For example, manufacturing operations for aluminum, magnesium, electronics, and electrical transmission and distribution equipment account for a significant portion of F-gas emissions.
Impact on Climate Change
F-gases, unlike methane and CO2, are not absorbed naturally. Their only natural sink is the atmosphere, where they combine with other gases and disperse around the earth. Once they reach the far-high atmosphere, they can survive for thousands of years before sunlight kills them.
Scientists are researching solutions to capture and repurpose these harmful greenhouse gasses.
Fluorinated gases are often emitted in smaller quantities than other greenhouse gases, yet they are very effective greenhouse gases. Global warming potentials (GWPs) range from thousands to tens of thousands, and they are also known as high-GWP gases because they trap significantly more heat per unit mass than CO2.
Hydrofluorocarbons
Hydrofluorocarbons (HFCs) are synthetic gases commonly used for cooling and refrigeration. Many HFCs are extremely potent, short-lived climate pollutants with an average atmospheric lifetime of 15 years.
Though HFCs currently account for around 2% of total greenhouse gases, their influence on global warming can be hundreds to thousands of times larger than that of carbon dioxide (CO2) per unit mass.

Perfluorocarbons
Perfluorocarbons (PFCs) are man-made chemicals that contain only fluorine and carbon. They are typically colorless, odourless, non-flammable, and chemically unreactive at ambient temperatures.
PFC gases do not exist naturally in the atmosphere but are produced synthetically. The most important transportation pathway is emissions to the air from industrial processes. In Sweden, emissions are reported from larger operations within the metals production and processing sector (primary aluminum production).
Health consequences vary slightly amongst perfluorocarbons. Some PFCs may harm the liver and kidneys, while others might irritate the skin and cause significant eye discomfort.
Sulfur Hexafluoride
Sulfur hexafluoride, generally known as SF6, is a ‘greenhouse gas’ comparable to carbon dioxide (CO2) that has long contributed to global warming.
SF6 is a synthetic, odourless gas used in the power sector to ensure network safety and reliability. It is extremely stable, non-toxic, non-flammable, and electronegative, which means it will not generate any other compounds that would affect its state or utility.
In fact, it’s estimated that, over a 100-year period, SF6 is 23,500 times more effective at trapping infrared radiation than CO2,1, meaning that 1 kg of SF6 has the same impact as 23,500 kg of CO2. Once in the atmosphere, it has an atmospheric lifetime of 3,200 years, which means it can accumulate without degrading for millennia to come.
Nitrogen Trifluoride
Nitrogen trifluoride (NF3) is a colorless gas. It emits a musty, unpleasant odour. It can be a powerful oxidant at high temperatures. Nitrogen trifluoride is a poisonous chemical that is most dangerous when inhaled. It causes the synthesis of methemoglobin, which lowers oxygen levels in the body’s tissues; however, exposure to fresh air or breathing oxygen converts it back to hemoglobin. The OSHA allowable exposure limits are defined at (Threshold Limit Values) TLV-TWA (Time weighted average) 10 ppm or 29 mg/kg. It is not corrosive to steel, stainless steel, or nickel if the metal surface has been thoroughly cleaned, passivated, and kept moisture-free.
Conclusion
Finally, It is crucial to understand that not all regions produce the same amounts of greenhouse gasses. As a result, each country must undertake its own set of policies to combat climate change. Scientists are sure that global temperatures will continue rising for decades, primarily due to human-caused greenhouse gas emissions. The environment has already been impacted by global climate change. Glaciers have receded, ice on rivers and lakes has melted, plant and animal habitats have shifted, and trees are blooming earlier. It’s worth learning about the most dangerous pollutants to make informed decisions and protect our health from harmful compounds. In conclusion, we should spread awareness about climate change due to greenhouse gasses.