NITROGEN OXIDES
Meet nitrogen, commonly referred to by the chemical symbol N. When two nitrogen atoms bond together, they form nitrogen gas (N₂), which makes up about 78% of the air in the atmosphere. Nitrogen and oxygen combine during fossil fuel combustion to form nitrogen dioxide (NO₂), a significant air pollutant. NO₂ is produced when high temperatures in combustion processes cause nitrogen from the air to react with oxygen, leading to harmful impacts on air quality. NO₂ is mostly released from cars, trucks, and power plant sources we encounter daily. NO₂ is also one of six “criteria pollutants” regulated under the Clean Air Act, which sets national air quality standards to protect public health and the environment.
Nitrogen dioxide (NO₂) significantly worsens urban air quality, especially affecting individuals with asthma or respiratory issues. It also contributes to the formation of ground-level ozone and fine particles, further degrading air quality. Nitric oxide (NO) is naturally produced by lightning and soil microbes, but the primary sources are combustion processes, where atmospheric oxygen and nitrogen combine in flames. While NO is produced within the human body, it is not emitted by humans.
NOx, apart from affecting human health and the environment itself, has the potential to produce “secondary pollutants” such as ozone, smog, nitric acid, and particulate matter, which have adverse effects on health and the environment. NOx monitoring is an efficient way to prevent the accumulation of high levels of NOx as it helps detect the amount of NOX we breathe in and alerts us when a certain level is exceeded. This article covers nitrogen oxides in the air, their sources, permissible levels in the ambient air, health, environmental impact, possible corrective measures, the need for nitrogen oxide monitors, and different methods of NOx monitoring. It’s about understanding a critical factor in air quality.
1. What is NOx?
In atmospheric chemistry, NOx stands for nitrogen oxides, mostly referring to two gases: NO (nitric oxide), which is invisible and odorless, and NO2 (nitrogen dioxide), which has a reddish-brown color and a sharp smell; and both are key pollutants. Nitrogen oxides such as NO3 (nitrogen trioxide) and N2O (nitrous oxide) are powerful greenhouse gases that harm the ozone layer. N2O4 is in equilibrium with NO2, while N2O5 is unstable and primarily found at night as it is rapidly broken down by sunlight. Nitrogen oxides (NOx) are a significant and intriguing group of air-polluting chemical compounds. However, the primary pollutants of concern are nitric oxide (NO) and nitrogen dioxide (NO2) which are explained below.
2. What’s the difference between NOX, NO and NO2?
- The phrase “nitrogen oxides” (NOx) refers to both nitrogen dioxide (NO2) and nitrogen monoxide (NO).
- Nitrogen monoxide (NO) is a colorless gas and one of the primary oxides of nitrogen.
- Nitrogen dioxide (NO2) is a reddish-brown gas with a unique, acidic odor. It is one of several nitrogen oxides.
Nitric Oxide: Nitrogen monoxide (NO), often known as nitric oxide, is a colorless gas with a strong, sweet odor and a hazardous air pollutant. It consists of one nitrogen atom bonded to one oxygen atom. Due to one unpaired electron, it is highly reactive and thus rapidly oxidized (within a few minutes) to NO2.
Nitrogen Dioxide: Nitrogen dioxide (NO2) is a critical air pollutant highlighted by air quality guidelines like those from the World Health Organization. NO2 is a reddish-brown acidic gas with a pungent, dissolves in water, and acts as a strong oxidant and corrosive. It forms when nitric oxide (NO) oxidizes during combustion, such as in diesel engines and power plants burning coal, oil, gas, wood, or waste. NO2 gas itself is not flammable. However, it can accelerate the burning of combustible materials.
Nitrogen oxides in the Atmosphere:
NOX is typically made from the reaction of nitrogen and oxygen when fossil fuels such as coal, oil, gas, or diesel are burned at high temperatures. Of the nitrogen compounds released during combustion, 10% is NO2, while 90% is NO. Once released in the atmosphere, Nitrogen dioxide can combine with other atmospheric chemicals to generate acid rain, harming ecosystems and human-built monuments, structures, and cultural sites. Nitrogen oxides, including NO₂, produce nitrate particles that cause haze and reduced visibility. Elevated NO₂ levels can cause environmental damage, including nutrient pollution in coastal waters.
High NOx levels can harm vegetation by increasing its susceptibility to disease and frost damage. When NOx combines with other pollutants under sunlight, it produces ozone (another critical air pollutant), which, in high amounts, also harms vegetation. Therefore, during the day, NO, NO2, and ozone (O3) exist in a quasi-equilibrium depending on the amount of sunlight. Moreover, Nitrogen oxides are essential contributors to photochemical smog, responsible for it’s yellowish-brown color.
The atmospheric chemistry in the areas where vehicular emission is a predominant source generally follows the trend given below:
- There is often a higher concentration of NO2 during peak traffic times.
- The concentration of O3 increases during the day as the sunlight rises, while the NO2 concentration decreases. This happens because NO2 reacts in the presence of the sun to form O3.
- In the late evening, NO2 concentrations build up as no sunlight converts NO2.
The remaining NO2 eventually reacts with other substances in the air to form particulate matter, gaseous nitric acid (HNO3), and toxic organic nitrates such as PANs (peroxyacetyl nitrates). However, NO2 may travel hundreds of kilometers before eventually converting to nitric acid or nitrates. It has a relatively short lifetime (about a day) and is concentrated near its sources.
3. Sources of NOx
Now that you’ve had a formal introduction to NOx, NO, and NO2, how are nitrogen oxides formed, and where do they come from? Ever wonder how nitrogen oxides are naturally produced? Well, it happens during lightning strikes! A single bolt can heat up to 30,000 kelvins (about 53,540 degrees Fahrenheit). Lightning strikes Earth around 100 times every second, with 86,400 seconds in a day and 365 days in a year. You can imagine that there is a ton of lightning!
NO2 is prevalent in the environment in small quantities, but due to our excessive use of resources and other activities, nitrogen dioxide levels are rising to a dangerous level. Some of the common Natural and man-made sources of NOx are listed below:.
- Volcanic eruptions and active volcanic locations.
- Possible sources of fertilizer include volcanic eruptions and the biological degradation of garbage.
- Lightning strikes increase the formation of NO2 gas.
- Car, truck, boots, and airplane emissions.
- Power plants and ammonia emitting fertilizer.
- Diesel-powered heavy machinery.
- Kerosene and gas stove.
4. Permissible levels of NO2
Below are the breakpoint concentrations describing air quality based on nitrogen dioxide concentrations for different countries. In India, daily NO2 levels of up to 80 µg/m3 are considered satisfactory.
Table: Breakpoints of nitrogen dioxide (µg/m3)
|
India (24-hr) |
US (24-hr) |
China (24-hr) |
EU (8-hr) |
||||
|
AQI Category |
Breakpoint concentration |
AQI Category |
Breakpoint concentration |
AQI Category |
Breakpoint concentration |
AQI Category |
Breakpoint concentration |
|
Good |
40 |
Excellent |
40 |
Very low |
50 |
||
|
Satisfactory |
80 |
Good |
80 |
Low |
100 |
||
|
Moderately polluted |
180 |
Lightly Polluted |
180 |
Medium |
200 |
||
|
Poor |
280 |
Moderately Polluted |
280 |
High |
400 |
||
|
Very Poor |
400 |
Very Unhealthy |
2260 |
Heavily Polluted |
565 |
Very high |
400+ |
|
Severe |
400+ |
Hazardous |
3760 |
Severely Polluted |
565+ |
||
[Source: National Ambient Air Quality Index, CPCB (Oct 2014)]
The World Health Organization (WHO) has updated its air quality guidelines to better protect public health. The new NO2 guideline is set at 10 µg/m³ as an annual average, marking a significant reduction of 30 µg/m³ from the previous guideline established in 2005. This update underscores the WHO’s commitment to enhancing global air quality standards to safeguard public health.
The table below will represent WHO Indoor and Outdoor Air Quality Guidelines for the NO2
|
Source |
Indoor Air Quality |
Outdoor Air Quality |
|
NO2 |
1 Hour = 200 µg/m3 Annual = 40 µg/m3 |
24 Hour = 25 µg/m3 Annual = 10 µg/m3 |
Source – National Library of Medicine
WORKPLACE EXPOSURE LIMITS for NO2
- OSHA: The legal airborne permissible exposure limit (PEL) is 5 ppm, not to be exceeded at any time.
- NIOSH: The recommended airborne exposure limit is 1 ppm, which should not be exceeded at any time.
- ACGIH: The recommended airborne exposure limit is 3 ppm averaged over an 8-hour workshift, and 5 ppm as a STEL (short-term exposure limit).
*Nitrogen Dioxide may cause mutations. All contact with this chemical should be reduced to the lowest possible level
(Source- Hazardous substances fact sheet)
5. Health & Environmental Impact of NOx
NO2 interacts with O2 to produce nitric acid, which causes corrosion and contributes to the development of other pollutants such as smog, PM, and acid rain. It is a flame accelerator but is not flammable.
Health impact
How does the presence of nitrogen oxides affect me? Are there any negative impacts on my health, and should I be concerned? Below, we will discuss the health impact of NOx.
- Prolonged exposure to nitrogen dioxide might cause severe respiratory issues.
- When inhaled, nitrogen dioxide irritates the eyes, nose, and throat, causing shortness of breath and long-term respiratory difficulties due to decreased lung capacity.
- For people with pre-existing respiratory conditions such as asthma, exposure to nitrogen dioxide can aggravate symptoms and trigger asthma attacks.
- NOx is one of the asphyxiant gases that cause deterioration in health by depriving the body of oxygen.
- Higher nitrogen dioxide levels have been related to an increased risk of developing chronic respiratory disorders such as bronchitis and chronic obstructive pulmonary disease (COPD).
- The mental health concerns investigated included common mental disorders (CMD), sleep apnea, anxiety, depression, and suicide.
It should be noted that nitrogen dioxide concentrations in indoor air can increase due to human activities such as heating and cooking, both of which emit nitrogen dioxide. If the indoor air is poorly ventilated, the gas can build up, causing very harmful effects to people who spend time indoors daily.
Below are Stats for the US, which show economic and health impact in the US
A study specifically examining the impacts of nitrogen dioxide exposure found that in areas with just a 5.9 parts per billion increase in pollutant concentration, there was
- A 22% increase in emergency room costs
- A 5% increase in outpatient costs,
- A 7% increase in annual direct healthcare costs
Environmental Impact
The presence of nitrogen dioxide causes a variety of issues in ecosystems. It can contribute to the acidity of soil and water, affecting aquatic plants and fauna. Furthermore, high amounts of nitrogen dioxide in the atmosphere can produce oxidative stress in plants, impairing their growth and vegetative development. The presence of significant levels of nitrogen dioxide in the atmosphere can disrupt the chemical equilibrium of the air, affecting global climate.
Nitrogen dioxide can also combine with other air pollutants to produce hazardous particulate matter. It also reacts with ozone, producing photochemical smog. This haze is not only bad for human health but may also impair ecosystems by limiting visibility and destroying flora.
Terrestrial Ecosystem: High NOx concentrations negatively impact crops, vegetation, and nutrient balance, leading to yield losses, eutrophication, acidification, increasing drought susceptibility, diseases, pests, and declining plant species diversity.
Aquatic Ecosystem: Eutrophication is the process by which a body of water becomes too nutrient-rich, resulting in the abundant proliferation of basic plant life. It is caused by Nitrogen oxides and ammonia. Increased absorption of NOx in water bodies upsets the chemical balance of nutrients, leading to oxygen depletion.
Nitrogen oxides can lead to the acidification of soils and bodies of water, disrupting ecosystems and leading to biodiversity losses. In short, nitrogen dioxide can significantly impact ecosystems, and it is important to reduce its presence in our air to protect our health and the health of our planet.
6. Possible corrective measures
The major preventive action is to monitor the levels of NOx, for which our Oizom air quality monitors are very effective. Aside from that, certain preventative methods could help us stay safe from the respiratory effects of this gas:
- Vehicles with low emission rates can help us limit the production of NOx.
- Alternative fuels, such as hydrogen cells and electric vehicles, can help to reduce NOx emissions.
- Avoid traffic congestion. Improving engines’ efficiency. Breathing such polluted air daily also leads to health effects in the long run.
- Have a walk, use bicycles, public transport, or carpool whenever possible.
- Breathing such polluted air daily also leads to health effects in the long run.
- Also, limit outdoor activities and close windows and vents during high air pollution.
- Industries can use low-NOx burners, specifically industrial and utility boilers, to lower NOx emissions.
- A confined environment is used to create fertilizer and process the gasses produced within it.
- A study found that the Lauraceae family plant Glutinosa species reduces NOx in the atmosphere and can be planted near sources of NOx emissions (Highways and roadways).
7. Understanding Nitrogen Dioxide Health Advisory Levels
|
Levels |
(µg/m3) |
Health Effects |
|
Good |
0-40 |
Fresh Air |
|
Satisfactory |
41-80 |
Coughing, difficulty in breathing experienced |
|
Moderately Polluted |
81-180 |
Breathing difficulties, aggravation of asthma |
|
Poor |
181-280 |
Reduced brain functionality and lung function alterations |
|
Very poor |
281-400 |
Brain damage and heart failure |
|
Severe |
400+ |
Life-threatening |
Source -NAQI as per CBCB. 2-h hourly average values
8. Measurement methods of NOx monitoring
There are several well-established monitoring methods for NO2, some of which can only measure NO2, while others can also measure NO and/or NOx. Chemiluminescence, cavity-attenuated phase shift spectroscopy (CAPS), semiconductors, and electrochemistry are different working principles for nitrogen oxide monitoring in the ambient environment.
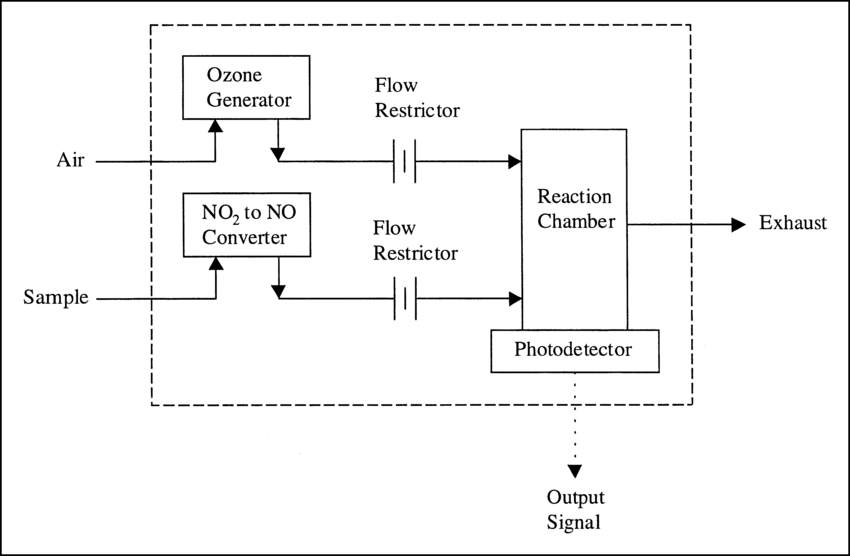
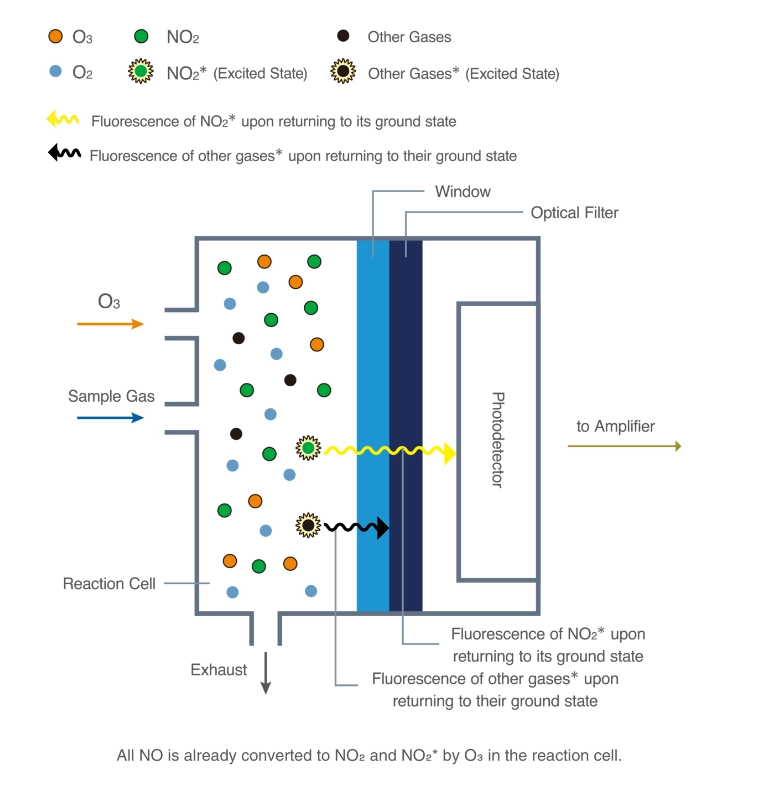
Chemiluminescence for NOx monitoring is the most commonly used conventional method of measuring the NOx levels in the air. It is based on a chemiluminescent reaction between nitric oxide (NO) and ozone (O3). In the NOx monitor, the atmosphere is drawn into two paths. In the first path, the NO2 in the sample is quantitatively reduced to NO using a converter. This converted NO2 and NO in the air reacts with ozone (produced utilizing an ozone generator) to generate activated NO2. During the conversion, light energy at a specific wavelength is proportional to the amount of NO+NO2 in the air sample. In the second path, the air directly reacts with O3, i.e., without passing through the reactor, and the light energy produced is directly proportional to the NO present in the air. The light intensity is measured and recorded photometrically from both paths. The signal from the second path is the amount of NO present in the air sample, while the electronic difference from both paths is the amount of NO2 present in the air sample.
This principle is used for continuous concentration measurement of nitrogen oxides (NOx: NO + NO2), NO, NO2, and ammonia (NH3) in sample gases.
[Source: https://www.researchgate.net/figure/Typical-chemiluminescence-analyzer_fig2_10689913]
Figure – Structure and operating principle of a gas analyzer using the chemiluminescence method.
Source- https://www.horiba.com/int/process-and-environmental/measuring-principles/chemiluminescence/
Cavity Attenuated Phase Shift Spectroscopy (CAPS) for NO2 monitoring – The NO2 monitor working with the CAPS principle operates as an optical absorption spectrometer that measures NO2 directly. Measurement is carried out in a temperature-controlled optical cell containing highly reflective mirrors on both sides to provide extensive optical path length, a blue UV light-emitting diode (LED) centered at 450 nm, and a vacuum photodiode detector. The NO2 present in the air drawn into the NO2 monitor causes a phase shift in the signal received by a photodetector proportional to the NO2 concentration.
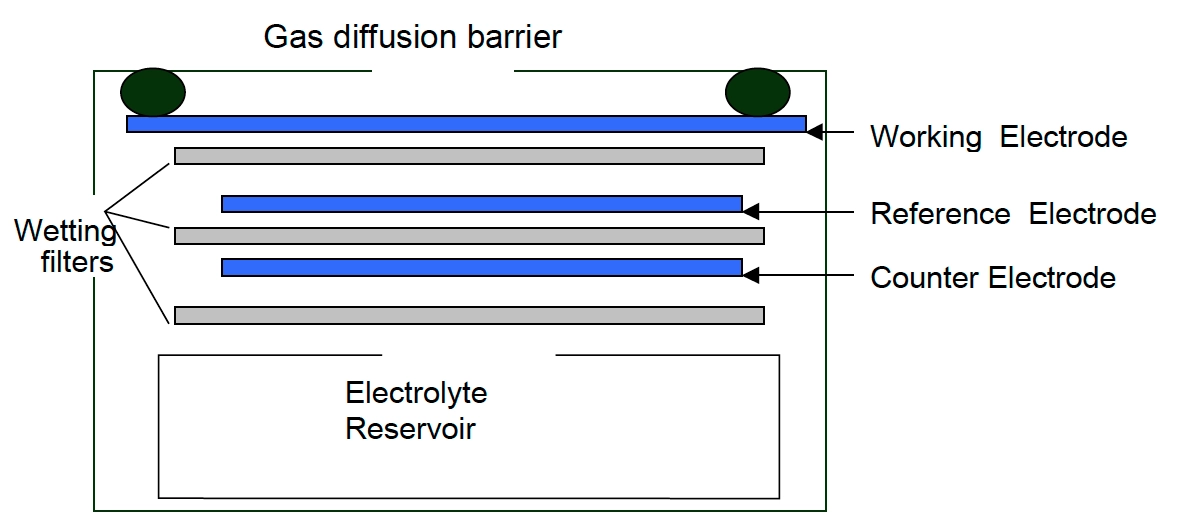
Electrochemical method for NOx monitoring – NOx monitors working on the electrochemical principle are operated based on the diffusion of nitrogen dioxide and nitric oxide gas into the respective sensor, producing electrical signals proportional to the NO2 and NO concentration, respectively. It allows accurate measurement of even low concentrations of NOx, which is essential in NO2 and NO monitoring for the ambient air.
[Source: https://docs.smartcitizen.me/Components/sensors/Electrochemical%20Sensors/]
Solid-State Metal Oxide Sensors
Metal Oxide Sensors are a prominent sensor technology for measuring NOx gas concentrations. Metal Oxide Sensors comprise a heating and semiconducting metal oxide sensing elements. The heater heats the sensor element’s surface to 300°-500°C, allowing it to detect gases via a chemical reaction on its surface. This reaction alters the electrical conductivity of the sensing element, which may be measured using an external circuit to determine the observed gas level.
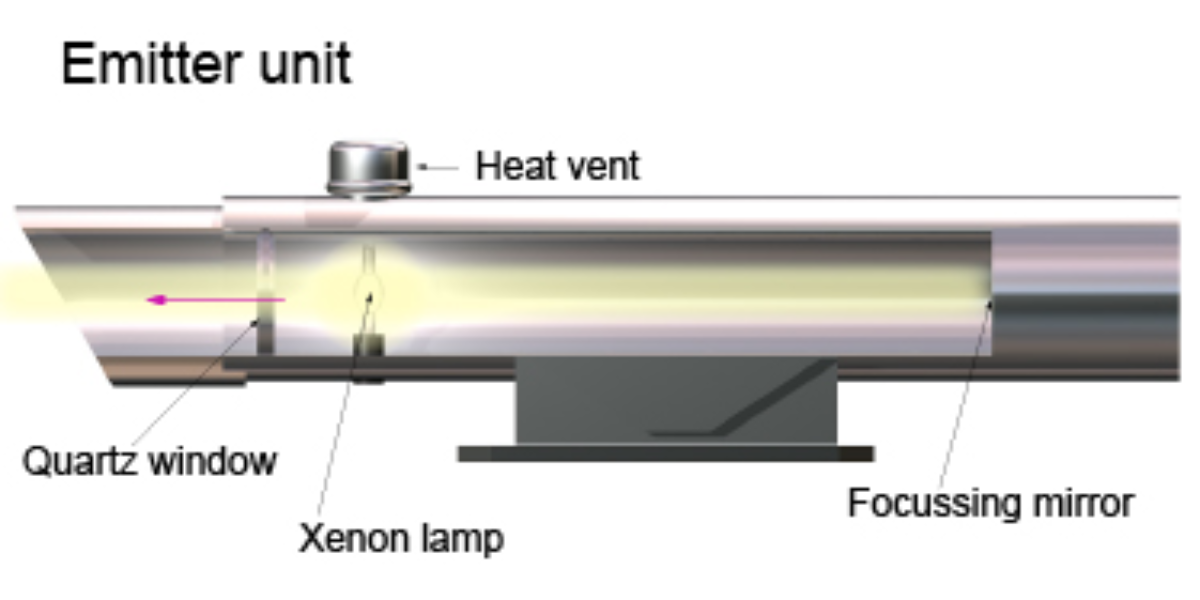
DOAS System (Differential Optical Absorption Spectroscopy)
The Differential Optical Absorption Spectroscopy (DOAS) system operates by measuring the absorption of light at specific wavelengths as it passes through a column of air. It consists of an emitter, receiver, and analyzer. The emitter projects light, including visible, ultraviolet, and infrared wavelengths, across a path of several hundred meters. The amount of light absorbed correlates with the concentration of pollutants, based on Beer-Lambert’s Law.
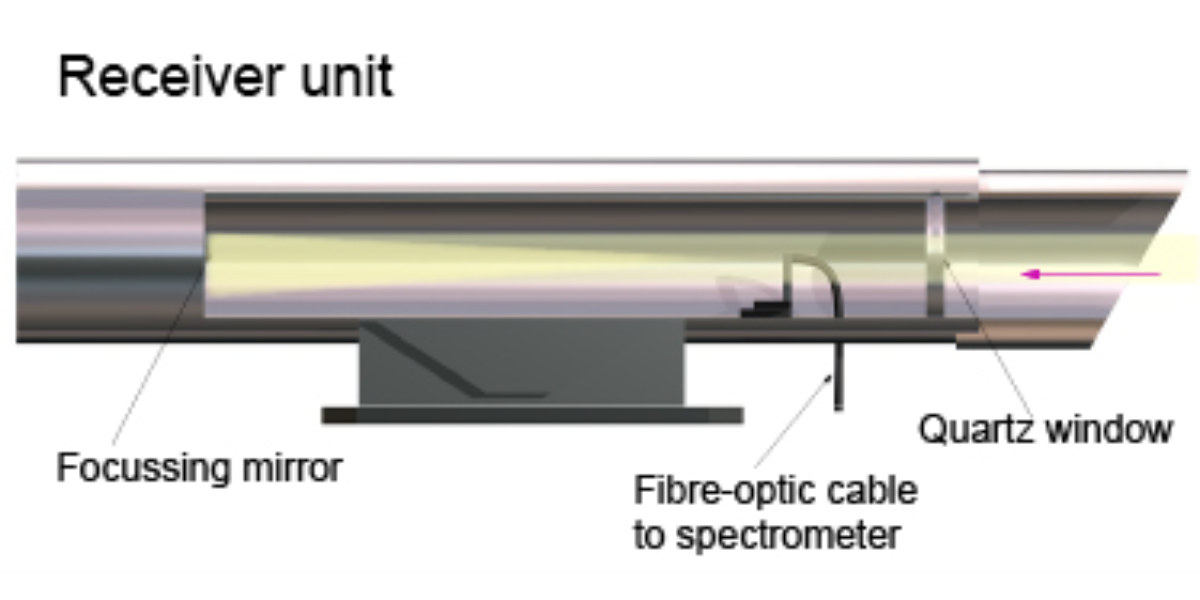
The receiver captures the transmitted light and directs it to the analyzer. The analyzer uses a spectrometer to split the light into narrow wavelength bands and compares the absorption data with reference spectra. This process allows for the simultaneous detection and quantification of multiple gases by analyzing their unique absorption features.
This system provides high-precision pollutant measurements in real-time, making it effective for monitoring air quality across large distances.
Illustration of a Differential Optical Absorption Spectroscopy emitter unit
Illustration of a Differential Optical Absorption Spectroscopy Receiver unit
9. Oizom’s sensor working principle for NOx monitoring
Oizom provides a range of nitrogen dioxide (NO2) and Nitric Oxide (NO) sensors modules to monitor varying NO2 and NO levels based on your needs. Our sensors accurately measure NO2 and NO in ambient conditions, detecting concentrations in ppb/ppm. This sensor monitors nitrogen dioxide and nitric oxide in real time. This sensor is integrated into a metal casing and ultra-low-noise support electronics, making it compact and reliable. This allows accurate gas detection even at very low concentrations in the atmosphere. This sensor works on the Electrochemical working principle to measure environmental air quality.
This sensor undergoes calibration using standard gases and tools to ensure high sensitivity and accuracy. Each gas sensor is calibrated with zero and span checks in a controlled lab, following Section 12.2 of the USEPA Quality Assurance Handbook for Air Pollution Measurement Systems Volume II. Calibration is done using NIST-traceable gas standards for reliable performance.
The NO2 and NO sensor module is integrated into outdoor air quality monitoring systems like Polludrone, AQBot, and Odosense. It is ideal for campus monitoring, smart cities, industries, wastewater treatment plants, roads, research projects, and environmental impact assessments. By utilizing this sensor module, users can ensure they receive accurate, real-time data on NO2 and NO levels, aiding in the effective monitoring and management of air quality across diverse environments.
10. Why Choose Oizom NO2 and NO Sensor?
- Compact: Our sensors are small and easy to install, perfect for use in any space, making them ideal for portable air quality monitoring. The NO2 and NO sensors come pre-calibrated and can be quickly replaced in just a few minutes by removing and replacing the old sensor with a new one.
- Durable: The NO2 and NO sensors have a long life of almost two years.
- Energy Efficient: There is no need to be concerned about energy usage because our sensors are not only accurate but also energy efficient. Powered up with just 3.3 to 5V for efficient, reliable performance!
- In-house sensor tech: Oizom applies advanced data processing algorithms to compensate for the effects of temperature and humidity on the sensor output. The algorithm is designed to automatically update based on weather conditions and seasonal changes, removing its influence on the sensor performance. The advanced algorithms also compensate for the effect of the cross-sensitive gas.
- Ultra-Low Noise Electronics: The sensor module’s design is crucial for accurate measurements. Each sensor is housed in a metal casing with ultra-low-noise electronics and a base PCB. The metal casing shields the sensor and electronics from electromagnetic interference, preventing false readings.
- RoHS Compliant: Our sensors comply with the RoHS criteria for restricting hazardous substances in electrical and electronic devices.
11. 5 Reasons why NO2 and NO monitoring is important
- NO2 and NO are major pollutants from vehicular emissions and energy production, resulting in poor air quality in almost all urban regions.
- NOx, apart from affecting human health and the environment itself, has the potential to produce “secondary pollutants” such as ozone, smog, nitric acid, and particulate matter, which have adverse effects on health and the environment.
- When inhaled, NOx causes damage to lung tissue and a reduction in the functioning of the respiratory system, making children, people with lung diseases such as asthma, and people who work or exercise outside more susceptible to adverse health impacts.
- NOx monitoring is an efficient way to prevent the accumulation of high levels of NOx. It helps detect the amount of NOX we breathe in and alerts us when a certain level is exceeded.
- Real-time monitoring of NOx levels helps calculate the air quality index, which is used to issue health advisories and formulate an action plan to meet standards.
FAQs
What is NOx, and why is it harmful?
NOx refers to a group of nitrogen oxide gases, mainly nitrogen dioxide (NO2) and nitric oxide (NO). These gases are harmful because they contribute to air pollution, leading to smog, acid rain, and respiratory problems in humans.
How does NOx affect the environment?
NOx gases react with other substances in the air to form harmful pollutants like ozone and fine particulate matter. This can damage ecosystems, harm wildlife, and contribute to global warming.
What are the main sources of NOx emissions?
The main sources of NOx emissions are burning fossil fuels, such as those used in vehicles, power plants, and industrial processes. These activities release NOx into the atmosphere, contributing to air pollution.
How can NOx emissions be reduced?
NOx emissions can be reduced by using cleaner energy sources, improving vehicle efficiency, and implementing pollution control technologies in industrial processes. Governments and industries are also setting stricter regulations to limit NOx emissions.
How is NOx measured?
NOx is measured using specialized air quality monitors like Oizom’s devices, such as polludrone, odosense, and AQbot. These monitors provide real-time data on NOx levels to track air quality and ensure regulatory compliance.
Is NOx heavier than air?
At high quantities, nitric oxide is rapidly oxidized in air to generate nitrogen dioxide exposures. Because nitrogen dioxide is heavier than air, it can cause asphyxiation in poorly ventilated, confined, or low-lying places.