Summary
Air quality plays a critical role in pharmaceutical manufacturing. From product safety and cleanroom integrity to worker health and regulatory compliance, keeping the air clean and controlled is non-negotiable.
This blog explored the key air quality parameters pharma facilities must monitor, like PM, VOCs, temperature, humidity, and pressure, and why traditional manual checks are no longer enough. With regulations like GMP, ISO 14644, and Annex 1 requiring continuous monitoring, real-time systems offer a smarter, more reliable solution.
We also looked at how Oizom’s Polludrone, a sensor-based, high-accuracy air quality monitor, is helping pharma companies like Alkem Laboratories achieve better control, faster responses, and audit-ready data.
Whether you’re from QA, EHS, or procurement, the need is clear: smarter air quality monitoring leads to safer processes, better compliance, and stronger outcomes.
Air Quality Monitoring in Pharma Industries
Clean air isn’t optional in the pharmaceutical industry. It’s the foundation of product safety and compliance. In pharmaceutical manufacturing, even a small change in air quality can affect drug quality, lead to contamination, or cause a batch to fail. From tiny particles in the air to changes in temperature or chemical vapors, every element matters.
Regulations like Good Manufacturing Practice (GMP), ISO 14644, and WHO guidelines exist for a reason: to ensure that the air inside cleanrooms and production zones meets strict safety and quality standards. But meeting those standards isn’t just about doing scheduled checks anymore.
Today, real-time air quality monitoring is becoming essential. It helps pharma teams catch problems early, stay audit-ready, and avoid costly downtime. Unlike manual sampling, these systems track key parameters like PM, VOCs, humidity, and pressure 24/7 and send instant alerts when something goes off.
This blog explains why air quality monitoring is so important in pharma, which areas need it the most, and how modern systems can support your team’s compliance and operational goals. If you’re part of quality, EHS, or procurement, and looking to improve your air monitoring setup, this is for you.
The Role of Air Quality in Pharmaceutical Manufacturing
Air quality directly influences product safety, employee health, and regulatory compliance in pharma facilities. Maintaining clean, controlled air isn’t just good practice. It’s essential to ensure consistent drug quality and prevent costly contamination risks.
Impact on Product Safety and Efficacy
In pharmaceutical manufacturing, air quality isn’t just part of the environment; it’s part of the process. Airborne contaminants like dust, microbial particles, and volatile organic compounds (VOCs) can directly compromise drug formulation, especially during critical stages like mixing, filling, and packaging.
Even the smallest particles can settle into open products, leading to variations in drug potency or unwanted reactions. This doesn’t just affect product quality; it puts patient safety at risk. That’s why cleanrooms are classified and controlled based on the maximum allowable particle count per cubic meter, as defined by ISO 14644.
If air quality isn’t tightly maintained, the risk of batch rejection increases significantly. Contamination-related recalls are not only costly but can damage the company’s credibility and regulatory standing. This is where continuous air monitoring offers real value; it gives teams real-time data to act before small issues become big problems.
Employee Health and Facility Integrity
Operators and technicians working in production zones are often exposed to active pharmaceutical ingredients (APIs), solvent vapors, and fine particulates. Without proper air control, prolonged exposure can lead to serious health concerns.
Beyond health, poor air quality can also affect facility performance. Pressure differentials are critical to preventing cross-contamination between cleanroom zones. A drop in positive pressure or a spike in VOC levels can go unnoticed without continuous monitoring, impacting the entire environmental control strategy.
Maintaining optimal air quality is no longer just a QA task; it’s a shared responsibility across operations, safety, and compliance teams. Real-time systems are now the backbone of that effort.
Concerned about your employees’ health and the air quality inside your production area? Choosing the right air quality monitoring system can be confusing. That’s where Oizom’s Polludrone comes in.
Polludrone is a smart solution that monitors both particulate matter (PM) and harmful gases in real-time, giving you reliable and accurate air quality data to help you make informed decisions.
At Oizom, we take data accuracy seriously. Our Polludrone Smart has undergone rigorous field evaluations, including benchmarking by third-party validation programs like AQ-SPEC (Air Quality Sensor Performance Evaluation Center) by South Coast AQMD.
Regulatory Compliance and Standards to Follow
Air quality in pharmaceutical manufacturing isn’t just about operational efficiency; it’s a regulatory necessity. To ensure product integrity and patient safety, global authorities have set strict standards that pharma facilities must meet, monitor, and document.
Key Guidelines for Air Quality in Pharma
- Good Manufacturing Practices (GMP): GMP guidelines form the foundation of pharmaceutical production. They require manufacturers to control environmental factors, including air quality, to avoid contamination and ensure reproducibility. This includes the need for validated HVAC systems, HEPA filtration, and regular environmental monitoring.
- ISO 14644 for Cleanrooms: ISO 14644 defines a cleanroom guide, which is classified based on the number and size of particles allowed per cubic meter of air. For example, a Grade A cleanroom (ISO Class 5) permits no more than 3,520 particles ≥0.5 µm per m³. This standard helps maintain the integrity of processes like aseptic filling and sterile compounding.
- WHO TRS 961 Annex 8: The WHO’s technical guidelines offer a global framework for HVAC system design and validation in pharmaceutical plants. Annex 8 emphasizes airflow control, pressure differentials, and particulate monitoring, critical for facilities operating in developing markets or under WHO prequalification.
- US FDA & EU Annex 1 Expectations: Both regulatory bodies require documented control of environmental parameters, especially in sterile manufacturing. Annex 1 (EU) now mandates continuous environmental monitoring for critical zones, reinforcing the need for real-time systems. The FDA’s focus lies on data integrity, system validation, and audit-ready reporting.
Following these standards isn’t optional. It’s the foundation for inspection readiness, market approval, and ultimately, patient safety.
Critical Parameters That Must Be Monitored
Effective air quality monitoring in pharmaceutical environments depends on tracking the right parameters. It’s not just about measuring what’s in the air; it’s about understanding how these factors impact product quality, regulatory compliance, and cleanroom performance.
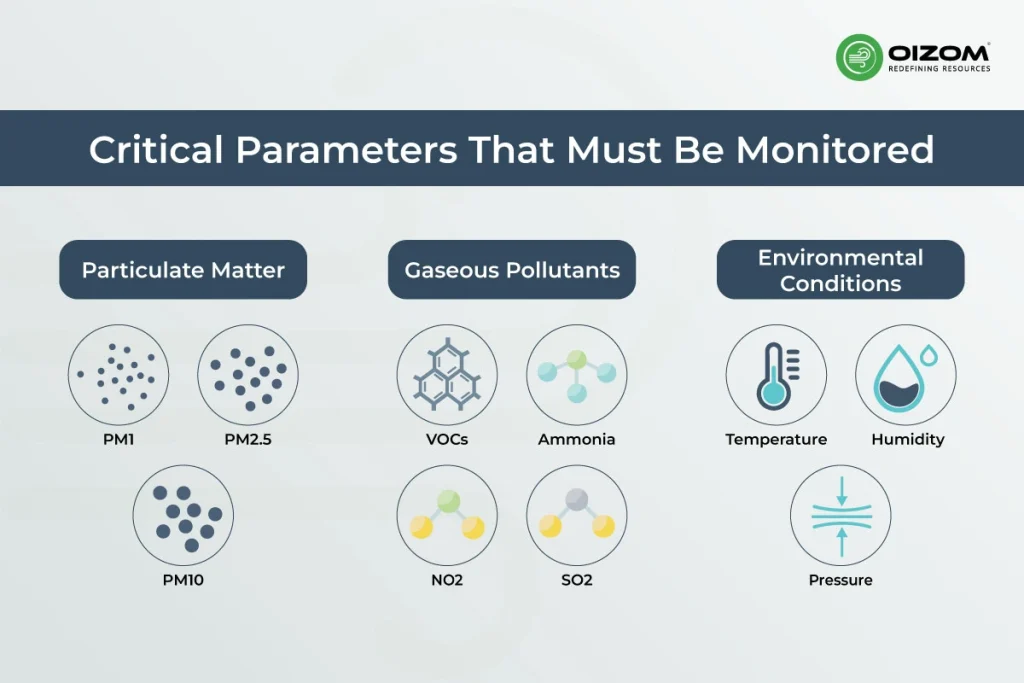
Particulate Matter
PM1, PM2.5, and PM10 are key indicators of airborne contamination. These particles can originate from raw materials, equipment movement, human activity, or HVAC systems. In cleanroom operations, even particles smaller than 2.5 microns can compromise sterile conditions and contaminate open product lines. Real-time PM monitoring helps maintain ISO class compliance and supports batch integrity.
Gaseous Pollutants
Volatile Organic Compounds (VOCs), ammonia, sulfur dioxide (SO2), nitrogen dioxide (NO2), and ethanol vapors are commonly present in pharma processes, especially in formulation, solvent handling, and cleaning stages. Without proper monitoring, these gases pose health risks to personnel and may also lead to chemical instability in sensitive products. Monitoring toxic gas concentrations ensures safe working conditions and helps maintain air exchange and filtration effectiveness.
Environmental Conditions
Temperature, humidity, and pressure differentials are critical for both process control and cleanroom classification. High humidity can affect hygroscopic ingredients, while pressure drops can allow contaminated air to flow into critical zones. Monitoring these parameters continuously ensures that HVAC and AHU systems maintain consistent conditions, reducing the chance of deviations and maintaining regulatory compliance.
By tracking these parameters together, facilities can build a stronger, data-driven environmental control strategy that supports both safety and quality.
High-Risk Zones in Pharma Facilities
Not all areas in a pharmaceutical plant carry the same level of risk. Some zones demand stricter air quality control due to their direct impact on product integrity and regulatory compliance. Understanding these high-risk zones helps in prioritizing monitoring and implementing the right level of environmental control.
Cleanrooms (Grade A to D)
Cleanrooms are the heart of sterile manufacturing. Each grade (A to D) has strict limits on particulate count and airflow specifications. Grade A, typically used for high-risk operations like aseptic filling, requires near-zero tolerance for airborne contaminants. Any fluctuation in particle levels, temperature, or pressure can disrupt the controlled environment. Continuous air quality monitoring is essential to meet ISO 14644 classifications and maintain sterility assurance.
API and Formulation Areas
These zones involve open handling of powders, solvents, and active pharmaceutical ingredients (APIs). They are high-risk due to the generation of both particulates and volatile gases. Poor air quality here can lead to cross-contamination or affect chemical reactions. Monitoring VOCs, PM levels, and maintaining proper ventilation ensures process stability and occupational safety.
Warehouses and Packaging Zones
Often overlooked, these areas can be a weak link in the air quality chain. Packaging zones handle exposed products or materials that are susceptible to airborne contamination. Similarly, poor air control in warehouses can introduce particulates that find their way into clean zones. Monitoring here ensures the final product stays protected before it reaches the market.
Advantages of Real-Time Air Quality Monitoring
Air quality issues can escalate quickly, especially in sensitive pharmaceutical environments. Relying on delayed data or missed trends increases the risk of non-compliance, product loss, and safety incidents. That’s where real-time monitoring bridges the gap between awareness and action.
Manual vs Automated Monitoring
Traditional air quality checks in pharmaceutical environments often rely on manual sampling, typically using filter-based methods at scheduled intervals. These readings are logged and reviewed later, often during routine inspections or audits. While they provide baseline data, manual methods have clear limitations: they are labor-intensive, subject to human error, and unable to capture real-time changes.
This creates blind spots. For example, a particulate spike during a shift change or a solvent leak during off-hours may go completely unnoticed. By the time a deviation is discovered, corrective action is often delayed, leading to potential product recalls, batch rejections, or regulatory non-conformance.
In contrast, automated, real-time monitoring systems offer continuous surveillance of critical parameters like PM, VOCs, temperature, humidity, and pressure differentials. These systems are designed to flag deviations instantly, helping EHS and QA teams act before problems escalate. They eliminate the need for manual logging, reduce reliance on periodic checks, and provide a reliable audit trail.
Automated systems also reduce operator exposure by minimizing the need to physically enter controlled zones for measurements. With integration into BMS or SCADA systems, corrective actions, like airflow adjustments or alarm triggers, can even be automated.
Simply put, manual monitoring tells you what happened. Real-time automated monitoring tells you what’s happening now, and that difference is critical in high-stakes pharma environments.
Benefits of Continuous Monitoring Systems
Real-time monitoring systems continuously track air quality parameters and immediately alert users to deviations. Whether it’s a sudden PM spike in a cleanroom or a rise in VOCs near an API formulation zone, these systems provide instant insights that support faster decision-making.
They also offer reliable data logging, making audit preparation smoother and more transparent. When integrated with SCADA, BMS, or PLC systems, monitoring becomes part of the broader automation strategy, enabling coordinated responses like airflow adjustments or process shutdowns.
Beyond compliance, real-time systems help identify patterns, reduce manual workloads, and cut long-term costs by preventing failures before they escalate. For pharma teams aiming to improve control, traceability, and efficiency, continuous monitoring is no longer optional; it’s essential.
Meet Oizom’s Polludrone, a sensor-based air quality monitoring system designed for high-precision, real-time tracking of ambient air conditions, making it ideal for CAAQMS applications in and around pharmaceutical facilities. It continuously monitors key environmental parameters such as PM1, PM2.5, PM10, VOCs, SO₂, NO₂, CO, and more, providing instant alerts when levels exceed defined thresholds.
What sets Polludrone apart is its data accuracy, validated through rigorous field trials and third-party benchmarking, including certification under South Coast AQMD’s AQ-SPEC program. It uses advanced correction algorithms and periodic calibration to ensure the reliability of its readings across diverse environments.
Use Case: How Oizom Supports Pharma
To understand the real impact of air quality monitoring in pharmaceutical facilities, let’s look at a real-world case scenario.
At Alkem Laboratories, maintaining clean air wasn’t just a compliance requirement; it was critical to ensuring product safety and protecting worker health. To support this, Oizom deployed its Polludrone Smart system for real-time air quality monitoring across key zones.
With its advanced sensor-based technology, Polludrone continuously tracks particulate matter, VOCs, temperature, and humidity, helping the EHS team detect deviations early and take corrective actions faster. This not only improved operational control but also strengthened their regulatory readiness.
This case shows how Oizom’s accurate, real-time monitoring solution can help pharmaceutical manufacturers maintain cleanroom integrity and support a safer, more efficient production environment. Read the full case study.
Conclusion
Maintaining clean, controlled air in pharmaceutical facilities isn’t just about ticking off compliance checklists; it’s about ensuring the safety, stability, and success of every product that leaves the line. As we’ve seen, poor air quality can impact everything from product efficacy to employee health and regulatory standing.
Real-time, sensor-based monitoring systems like Oizom’s Polludrone are helping pharma companies move from reactive fixes to proactive control. By continuously tracking key parameters like PM, gases, temperature, and humidity, facilities can detect issues early, respond faster, and stay audit-ready, all while improving operational efficiency.
Looking ahead, the role of air quality monitoring will only grow. With increasing regulatory scrutiny, greater emphasis on data-driven decision-making, and the rise of Industry 4.0 in pharma, continuous environmental monitoring will become a standard, not an exception.
For pharma leaders focused on quality, safety, and future-proofing their operations, now is the time to invest in smarter air quality solutions. Because in a field where precision matters, clean air isn’t just an input, it’s a commitment.
FAQs
Acceptable air quality in pharma cleanrooms is defined by ISO 14644-1 standards. For example, Grade A (ISO Class 5) allows no more than 3,520 particles ≥0.5µm per cubic meter. Each grade (A–D) has strict particulate limits to ensure product sterility and safety.
Air quality should be continuously monitored in critical zones, such as cleanrooms and API areas. While periodic sampling is still used, real-time monitoring is now preferred to catch deviations instantly and maintain GMP compliance.
Particulate matter (PM1, PM2.5, PM10) and gaseous pollutants like VOCs, SO₂, NO₂, ammonia, and ethanol are most harmful. They can lead to product contamination, chemical instability, and health risks for workers.
Yes. Real-time air quality monitoring systems offer continuous data logging, automated alerts, and audit-ready reports, making them more reliable and efficient than manual methods for meeting regulatory audit requirements.






