-
Chlorine gas (Cl2) may not be something you think about often, but it plays a surprisingly important part in our everyday lives. It is often used to treat our drinking water and is important in many industrial operations. While chlorine gas is useful, there is a downside that should be considered, particularly in terms of environmental impact.
When chlorine gas is released into the atmosphere, it does not simply fade; it can combine with other molecules, forming toxic compounds that are hazardous to the environment and our health. For example, if it enters water sources, it can harm aquatic life and destabilize ecosystems. Even though chlorine is useful for various purposes, it must be handled cautiously to avoid these undesirable consequences.
By being aware of how chlorine gas affects the environment, we can take steps to reduce its impact, like finding safer alternatives or improving how we manage it. This article covers information on chlorine gas, its sources in the ambient air, permissible levels, health and environmental impact, possible corrective measures, the need for chlorine monitors, and different methods of Cl2 monitoring.
1. What is Cl2?
Chlorine (Cl2) gas is a yellowish-green gas with a pungent odor used in various applications, including disinfection, water treatment, manufacturing, papermaking, the textile industry, bleaching, and pharmaceuticals. It is detectable at low concentrations (above 0.3 – 0.5 ppm). Cl2 is heavier than air, which causes it to remain in low-lying areas or near the ground with little air movement. It is a chemical element that belongs to the halogen group with the symbol Cl. It was discovered in the 1770’s and soon became useful as a commercial agent.
Chlorine gas is slightly soluble in water. Hence, it reacts to form hypochlorous acid (HClO) and hydrochloric acid (HCl). It is not flammable but can react explosively when combined with other substances such as hydrogen, ammonia, fuel gas, acetylene, ether, etc. Cl2 gas is oxidizing in nature and has bleaching properties. It is toxic to human and plant life and can corrode metals and other materials. Continuous or high-level exposure to Cl2 can quickly deaden a person’s sense of smell, making the odor of Cl2 an unreliable indicator of its presence. Hence, other means, such as chlorine monitoring, are viable solutions to provide adequate warnings of hazardous exposure.
Did you know this? Chlorine gas is about two and a half times heavier than air. It turns into a liquid at -34°C (-29°F). It has a strong, choking odor, and breathing it in can cause suffocation, chest tightness, and throat discomfort. In severe cases, it can lead to the lungs filling with fluid, which is extremely dangerous. Chlorine was the first gas used in chemical warfare in World War I.
Chlorine in Atmosphere:
Elemental chlorine is rarely present itself in nature due to its high reactivity. It can be formed from atmospheric reactions of chlorine-containing compounds with NO2 and through oxidation of chlorides in the presence of strong oxidants, such as ozone.
Once released, it rapidly combines with other chemicals/compounds in the atmosphere to form secondary compounds instead of remaining in a pure elemental state. Chlorine dissolves in the water and reacts to form chloride salts and chlorinated organic chemicals such as sodium chloride (NaCl, common salt), sodium hypochlorite (bleach), chloroform, etc.
Many chlorine-containing chemicals get released to the ground. Those that dissolve in water cannot reach substantial stratospheric heights because they are “washed out” of the atmosphere in rain or snow. For example, evaporated ocean spray releases huge amounts of chlorine in the form of sea salt (sodium chloride) particles. However, because sea salt dissolves in water, the chlorine is swiftly absorbed in clouds, ice, snow, or rain droplets and does not reach the stratosphere.
2. Sources
If you want to know the source of chlorine, you need to learn about the compounds that contain the chloride item. There are many different ways to get chlorine. It can be found in many different forms throughout nature. Because of its high reactivity, chlorine is unlikely to occur naturally in its free form. When asked what the source of chlorine in nature is, you can safely say Sodium Chloride. Chlorine can also be found as hydrochloric acid and ammonium chloride. Below, we will discuss some sources of chlorine.
- Chemical manufacturing: Chlorine is used to make organic chemicals like vinyl chloride monomer, ethylene dichloride, and chlorinated solvents.
- Pharmaceuticals: Chlorine is a key ingredient in drugs used to treat asthma, blood pressure, epilepsy, inflammation, and anemia
- Swimming pools and household bleach are two other sources of chlorine on the ground. When discharged, chlorine is quickly transformed into water-soluble forms and eliminated from the lower atmosphere. Such chlorine never reaches the stratosphere in large quantities.
- The exhaust from the Space Shuttle and some rockets inject chlorine directly into the stratosphere; the quantities are very small (less than 1% of the annual input from halocarbons in the present stratosphere).
- Even “typical industrial” organic chlorine compounds like pentachlorophenol, polychloropyrols, polychlorinated biphenyls (PCB), and tetrachlorodibenzodioxins (TCDD) have been identified as naturally occurring chemicals in 8,000-year-old sediments.
Were you aware of this? The oceans release about three million tonnes of methyl chloride, an organochlorine, into the atmosphere yearly, much more than humans produce.
3. Permissible exposure limits for Cl2
The permissible exposure limits of Cl2 in terms of continuous occupational exposure are given below:
Cl2 concentration
OSHA (PEL)
0.5 ppm TWA
OSHA (PEL)
1 ppm STEL
NIOSH (REL)
1 ppm STEL
NIOSH (IDHL)
10 ppm
AIHA ERPG-1
1 ppm
AIHA ERPG-2
3 ppm
AIHA ERPG-3
20 ppm
[Source: https://www.chlorineinstitute.org/stewardship/chlorine/health-hazards/]
Permissible Exposure Limit (PEL) given by OSHA (Occupational Safety and Health Administration) defines the maximum concentration of Cl2 to which an unprotected worker may be exposed. PEL may reference an eight-hour time-weighted average (TWA) or a 15-minute short-term exposure limit (STEL) concentration that cannot be exceeded for any period. Similarly, Recommended Exposure Limit (REL) and Immediately Dangerous to Life of Health (IDLH) are the occupational exposure limits recommended by NIOSH (National Institute for Occupational Safety and Health), and the Emergency Response Planning Guidelines (ERPGs) are exposure guidelines given by AIHA (American Industrial Hygiene Association).
Workplace Exposure Limits
- OSHA: The legal airborne permissible exposure limit (PEL) is 1 ppm, not to be exceeded at any time.
- NIOSH: The recommended airborne exposure limit (REL) is 0.5 ppm, which should not be exceeded during any 15-minute work period.
- ACGIH: The threshold limit value (TLV) is 0.5 ppm averaged over an 8-hour work shift and 1 ppm as a STEL (short-term exposure limit).
The above exposure limits are for air levels only. When skin contact also occurs, you may be overexposed, even though air levels are below the above limits.
4. Health & Environmental Impact of Cl2
Health Impact
Acute health effects from chlorine exposure may arise quickly, including:
- Chlorine contact can irritate and burn skin and eyes, perhaps causing eye injury.
- Contact with liquid chlorine can result in frostbite.
- Exposure might irritate the nose and throat, resulting in coughing and wheezing.
- Inhaling chlorine can irritate the lungs, resulting in coughing and shortness of breath. Increased exposure can lead to pulmonary edema, a medical emergency characterized by extreme shortness of breath.
- Exposure may result in headaches, dizziness, nausea, and vomiting.
The following chronic (long-term) health effects can occur at some time after exposure to Chlorine and can last for months or years:
- Cancer Hazard: While chlorine has been tested, its potential to cause cancer is not classifiable.
- Reproductive Hazard: chlorine has been tested, but its potential to disrupt reproductive systems cannot be determined.
Other Effects
- Chlorine can trigger an asthma-like allergy. Future exposure can trigger asthma attacks characterized by shortness of breath, wheezing, coughing, and/or chest tightness.
- Repeated exposure can induce bronchitis, which includes coughing, phlegm, and/or shortness of breath, and can result in chronic lung damage.
- Long-term contact can cause tooth damage, a skin rash, swelling and blisters, hoarseness, or loss of voice.
Some health effects associated with chlorine gas exposure are given below:
Cl2 levels
Health effects
1 – 3 ppm
Mild mucous membrane irritation
5 – 15 ppm
Moderate irritation of the respiratory tract.
30 ppm
Immediate chest pain, vomiting, dyspnea (shortness of breath), and cough
40 – 60 ppm
Toxic pneumonitis (inflammation of the lungs) and pulmonary edema (accumulation of fluid in the lungs)
430 ppm
Lethal over 30 mins
1000 ppm
Fatal within minutes
[Source: https://www.chlorineinstitute.org/stewardship/chlorine/health-hazards/]
Environmental Impact
Chlorine can enter the environment via the atmosphere as HCl gas and aerosol, or through chlorine gas for disinfection and defouling of water and sewage effluents, or as chloride ions (Cl-) in precipitation.
- Effects on Vegetation: Chlorine gas leaks from sewage treatment plants, railroad derailments, industrial disasters, and air pollution have been related to harm to vegetation.
- Effect of Acid Precipitation: Sulfate (from SO2 emissions) and nitrate (from NOx emissions) significantly contribute to atmospheric acidity. Acid deposition has been linked to global forest degradation (Hinrichson, 1987). Chlorine and HCl contribute significantly less to global acid deposition than sulfate and nitrate emissions.
- Effects on Animals: There is no available data on the health effects of past or current atmospheric chlorine exposure in animals. However, a study by Joyner and Durel in 1962 investigated the impact of a 6,000-gallon liquid chlorine spill on domesticated animals in La Barre, Louisiana.
5. Possible corrective measures
The primary action is Cl2 monitoring i.e., to measure how much of chlorine concentrations you are exposed to. In addition to this, the following corrective measures can be taken:
- Provide Traning: Workers who work with chlorine require training and supervision to ensure safety. (Refer to OSHA Standards 1910.1200 and 1910.119)
- Use Respiratory Protection: Ensure workers wear an approved self-contained breathing apparatus in areas where chlorine is stored or used. (See OSHA Standard 1910.134.)
- Prepare an escape plan: Prepare an emergency strategy in case of chlorine emissions. Remember to go uphill and upwind.
- Avoid going to or staying in low-lying areas near the source of Cl2.
- On the other hand, if the presence of Cl2 is detected, immediately vacate the area and provide proper ventilation to remove the gas.
- Avoid storing chlorine near gasoline or other combustibles. It can suddenly erupt and start a hazardous fire.
- Use chlorine in well-ventilated places. Ensure that eyewashes, showers, and oxygen are readily available. Provide access to self-contained breathing devices or canister respirators.
- Post emergency contact numbers in multiple areas across the facility and office. Provide contact information for the local fire department, police, and county emergency management office.
6. Measurement methods of Chlorine monitoring
Different working principles for chlorine monitoring in the ambient environment are pulsed NDUV (Non-Dispersive UV Absorption Spectroscopy), semiconductor, and electrochemistry.
Non-Dispersive UV Absorption Spectroscopy: The Cl2 monitor working on the principle of NDUV is based on the absorption of ultraviolet radiation at a particular wavelength. When the gas sample in the chlorine monitor is exposed to ultraviolet light, the UV radiation absorbed by the Cl2 molecules present in the gas sample is measured by the detector and converted to chlorine concentration.
Semiconductor – When a metal oxide semiconductor-based chlorine monitor is exposed to an air sample, the Cl2 molecules react on the metal oxide surface of the sensor and dissociate into charged ions that alter the film’s resistance. This interaction is measured as a signal and is converted to the gas concentration. However, the energy consumption of such Cl2 monitors is higher compared to others.
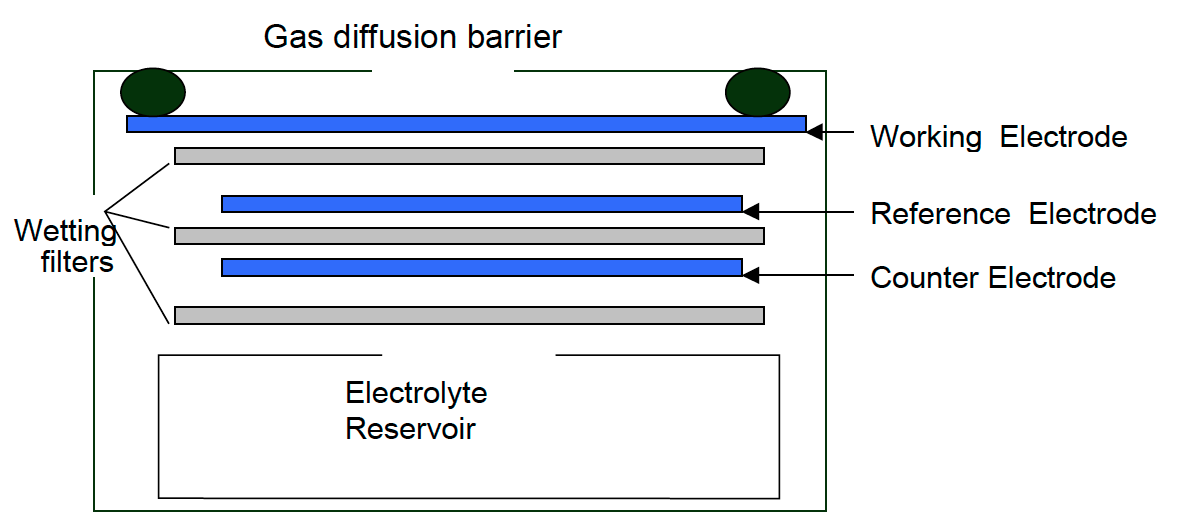
Electrochemical – Cl2 monitors working on the electrochemical principle are operated based on the diffusion of chlorine gas into the sensor, producing electrical signals proportional to the Cl2 concentration. It allows accurate measurement of even low concentrations of Cl2, essential in Cl2 monitoring in the ambient air.
[Source: https://docs.smartcitizen.me/Components/sensors/Electrochemical%20Sensors/]
Among all the above principles of Cl2 monitoring, chlorine monitors based on electrochemistry are typically preferred for ambient air monitoring as they yield more accurate Cl2 concentrations and are inexpensive compared to the others.
7. Oizom’s sensor working principle for Cl2 monitoring
Oizom provides a range of Chlorine (Cl2) sensor modules to monitor varying Cl2 levels based on your needs. Our sensors accurately measure Cl2 in ambient conditions, detecting concentrations in ppb/ppm. This sensor monitors Chlorine in real-time. This sensor is integrated into a metal casing and ultra-low-noise support electronics, making it compact and reliable. This allows accurate gas detection even at very low concentrations in the atmosphere. This sensor works on the Electrochemical working principle to measure environmental air quality.
This sensor undergoes calibration using standard gases and tools to ensure high sensitivity and accuracy. Each gas sensor is calibrated with zero and span checks in a controlled lab, following Section 12.2 of the USEPA Quality Assurance Handbook for Air Pollution Measurement Systems Volume II. Calibration is done using NIST-traceable gas standards for reliable performance.
The Cl2 sensor module is integrated into odor monitoring systems such as Odosense and AQBot, making it suitable for various applications, including disinfection processes, water treatment plants, pulp and paper industries, and food processing facilities. Its ability to provide accurate, real-time data on chlorine levels helps maintain safe and compliant operations in these industries.
8. Why Choose Oizom Cl2 Sensor?
- Compact: Our sensors are small and easy to install, perfect for use in any space, making them ideal for portable air quality monitoring. The Cl2 sensors come pre-calibrated and can be quickly replaced in just a few minutes by removing and replacing the old sensor with a new one.
- Durable: The Cl2 sensor has a long life of almost two years.
- Energy Efficient: There is no need to be concerned about energy usage because our sensors are accurate and energy efficient. Powered up with just 3.3 to 5V for efficient, reliable performance!
- In-house sensor tech: Oizom applies advanced data processing algorithms to compensate for the effects of temperature and humidity on the sensor output. The algorithm is designed to automatically update based on weather conditions and seasonal changes, removing its influence on the sensor performance. The advanced algorithms also compensate for the effect of the cross-sensitive gas.
- Ultra-Low Noise Electronics: The sensor module’s design is crucial for accurate measurements. Each sensor is housed in a metal casing with ultra-low-noise electronics and a base PCB. The metal casing shields the sensor and electronics from electromagnetic interference, preventing false readings.
- RoHS Compliant: Our sensors comply with the RoHS criteria for restricting hazardous substances in electrical and electronic devices.
9. Reasons why Cl2 monitoring is important
- Cl2 is a highly reactive, greenish-yellow gas with a suffocating, irritating, pungent bleach-like odor and can accumulate in enclosed, poorly ventilated, and low-lying areas.
- Cl2 reacts with moisture in the human body’s tissues and forms toxic products such as hydrogen chloride (HCl) and hypochlorous acid (HOCl) that cause damage to tissues and cell structure of the body.
- Exposure to chlorine gas may irritate the eyes, skin, and respiratory tract and cause coughing and wheezing. Accumulation of fluid in the lungs, etc, and may even lead to death. It also contributes to ozone layer depletion.
- Continuous or high-level exposure to Cl2 can quickly deaden a person’s sense of smell, making the odor of Cl2 an unreliable indicator of its presence. Hence, other means, such as using Cl2 monitors, are viable solutions to provide adequate warnings of hazardous exposure.
- Real-time Cl2 monitoring helps in determining the source of odor and formulating an action plan to control chlorine emission.
FAQs
- What is chlorine gas (Cl2)?
Chlorine gas (Cl2) is a highly reactive, greenish-yellow gas commonly used in water purification and industrial processes, with a strong, bleach-like odor. - How does chlorine impact the environment?
When released into the air, chlorine reacts with other chemicals, forming harmful compounds that can pollute water and harm ecosystems. - What are the health risks of chlorine exposure?
Inhalation of chlorine gas can cause breathing difficulties, chest tightness, throat irritation, and, in severe cases, lung damage. - What are the main sources of chlorine in the atmosphere?
Chlorine in the atmosphere comes from natural sources like oceans and volcanic activity, as well as human activities such as industrial processes and water treatment. - How can chlorine levels in the air be monitored?
Chlorine monitors detect Cl2 in the air, providing early warnings to prevent hazardous exposure and protect human health and the environment. - Is chlorine gas visible?
Yes, chlorine gas is mildly yellow-green, but visibility depends on concentration, lighting, and humidity. However, relying on visibility alone isn’t safe; its odor and potential harm require quick action. - How long does chlorine gas stay in the air?
Chlorine gas can remain for minutes to hours, depending on temperature, wind, and surface interactions like absorption by soil or water. - How do you test for chlorine gas?
- Odor: Detectable by its strong bleach-like smell.
- Litmus Paper: Blue paper turns red and white when exposed to chlorine.
- Chemical Kits: Use reagents to confirm their presence through color change.
- Gas Detectors: Provide accurate detection and monitoring.
- Real-Time Air Quality Monitoring: Oizom’s Odosense and AQBot monitor real-time chlorine (Cl2) levels, providing accurate air quality data to ensure environmental safety
Chlorine monitoring
Chlorine monitoring
All you want to know about Cl2


 [Source:
[Source: 
