Carbon monoxide (CO) is a gas you can’t see, smell, or taste, but it can be dangerous for us and the environment. It’s produced when things like gasoline or wood don’t burn completely, especially in places with a lot of traffic or industrial activity. Exposure to high levels of CO can cause CO poisoning, leading to serious health issues, giving it the name “The Silent Killer.” While CO is not the primary cause of global warming, it does contribute to disrupting the balance of greenhouse gases, potentially exacerbating climate change. Even small levels of CO can produce headaches and dizziness in humans, and prolonged exposure can be quite hazardous.
Monitoring and reducing CO emissions is essential for creating better, safer environments and tackling broader climate challenges. This article covers information on carbon monoxide, its sources, permissible levels in the ambient air, health and environmental impact, possible corrective measures, the need for carbon monoxide monitors, and different methods of CO monitoring.
1. What is Carbon Monoxide?
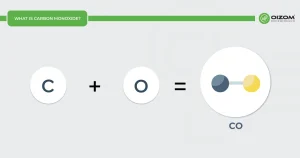
Carbon monoxide is a combustible gas without color, taste, or odour. It is the most common and harmful gas found in both indoor and outdoor environments. It is produced by the incomplete combustion of carbon-containing fuels such as natural gas, gasoline, or wood. It is emitted by various combustion sources, including automobiles, power plants, wildfires, and incinerators. Mobile sources account for the vast majority of outdoor CO emissions to ambient air nationally and in metropolitan regions.
Carbon monoxide can also be produced by photochemical reactions involving methane and non-methane hydrocarbons, other volatile organic hydrocarbons in the atmosphere, and organic molecules in surface waters and soil. Some indoor sources of CO also add to overall exposure. The molecular weight of carbon monoxide is similar to that of air: about 28.01 g/mole. Carbon monoxide consists of one oxygen atom and one carbon atom, which are further attached together by a triple bond consisting of two covalent bonds and one dative covalent bond. The chemical formula of carbon monoxide is given as CO. It is flammable and reacts vigorously with oxygen, acetylene, chlorine, fluorine, and nitrous oxide. It is slightly soluble in water, blood serum, and plasma.
Carbon Monoxide in Atmosphere:
CO is naturally present in the atmosphere at very low concentrations of about 0.2 ppm, which is not harmful to humans independently. CO is released naturally from volcanic eruptions, forest fires, etc. However, human activities are the biggest source of CO emissions, among which the largest proportion are emitted by the exhaust of internal combustion engines of motor vehicles running on petrol and diesel. It remains in the atmosphere for about two months and eventually reacts with oxygen to form carbon dioxide.
Carbon monoxide is a trace gas in the atmosphere that has no direct effect on global temperature, unlike methane and carbon dioxide. On the other hand, it plays an important part in atmospheric chemistry and impacts the atmosphere’s ability to cleanse itself of many other pollutants. In combination with other pollutants and sunlight, it contributes to the development of lower-atmospheric (“bad”) ozone and urban smog.
The levels of carbon monoxide are typically highest during winters as cold temperatures inhibit the combustion processes, resulting in higher CO formation. Additionally, the meteorological conditions during winters trap the pollutants near the ground. The use of gas furnaces and heaters increases in this season, adding to CO emissions.
2. Sources
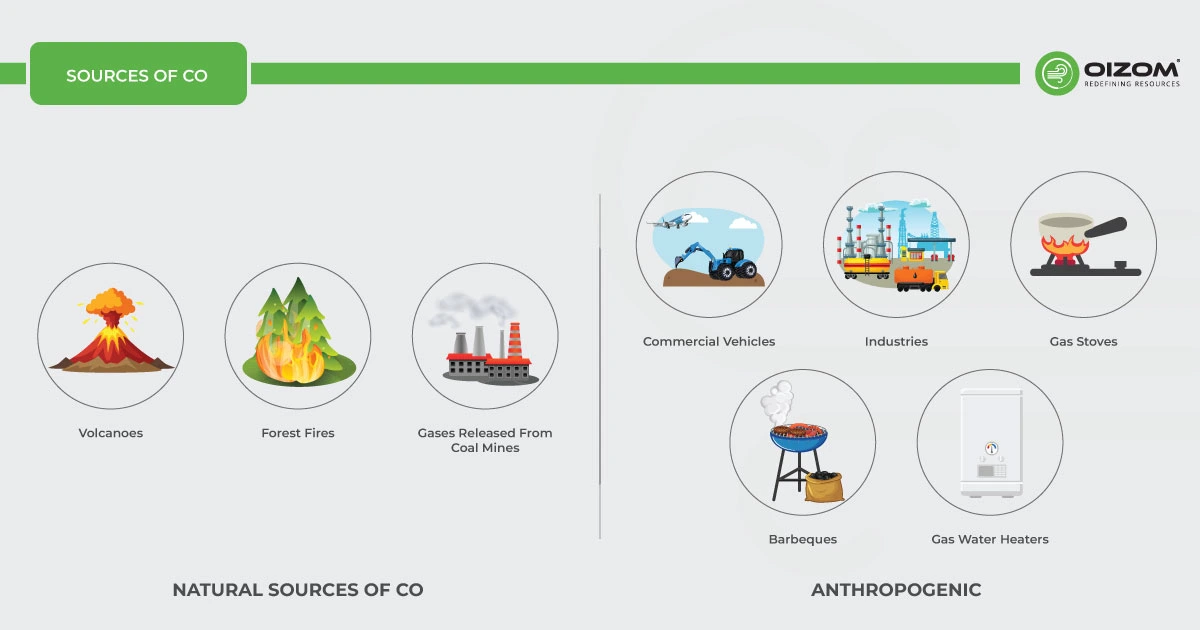
Carbon monoxide (CO) is a dangerous gas that can be inhaled in large quantities. It can be released from a variety of anthropogenic sources and also exists naturally.
Natural
Carbon monoxide is emitted from natural sources such as volcanoes, bush/forest fires, natural gases released from coal mines, the metabolism of animals, and even lightning.
Anthropogenic
- Combustion occurs in commercial vehicles such as cars, airplanes, tractors, and trucks.
- Industries working at high temperatures and using carbon compounds as raw products.
- Additional sources include breathing in tobacco smoke, burning biomass for cooking, equipment, or appliances such as heaters, barbeques, gas stoves, gas water heaters, fireplaces, Extracting gas from sea or land, etc.
3. Permissible Levels of Carbon Monoxide
The breakpoint concentrations describing air quality based on the carbon monoxide concentrations for different countries are given below.
Table: Breakpoints of carbon monoxide (mg/m3)
|
India (8-hr) |
US (24-hr) |
China (24-hr) |
EU (8-hr) |
||||
|
AQI Category |
Breakpoint concentration |
AQI Category |
Breakpoint concentration |
AQI Category |
Breakpoint concentration |
AQI Category |
Breakpoint concentration |
|
Good |
1 |
Good |
5 |
Excellent |
2 |
Very low |
5 |
|
Satisfactory |
2 |
Moderate |
11 |
Good |
4 |
Low |
7.5 |
|
Moderately polluted |
10 |
Unhealthy for sensitive |
14 |
Lightly Polluted |
14 |
Medium |
10 |
|
Poor |
17 |
Unhealthy |
18 |
Moderately Polluted |
24 |
High |
20 |
|
Very Poor |
34 |
Very Unhealthy |
35 |
Heavily Polluted |
36 |
Very high |
20+ |
|
Severe |
34+ |
Hazardous |
58 |
Severely Polluted |
36+ |
||
- OSHA PEL: The current Occupational Safety and Health Administration (OSHA) permissible exposure limit (PEL) for carbon monoxide is 50 parts per million (ppm) parts of air (55 milligrams per cubic meter (mg/m(3))) as an 8-hour time-weighted average (TWA) concentration [29 CFR Table Z-1].
- NIOSH REL: The National Institute for Occupational Safety and Health (NIOSH) has established a recommended exposure limit (REL) for carbon monoxide of 35 ppm (40 mg/m(3)) as an 8-hour TWA and 200 ppm (229 mg/m(3)) as a ceiling [NIOSH 1992]. The NIOSH limit is based on the risk of cardiovascular effects.
- ACGIH TLV: The American Conference of Governmental Industrial Hygienists (ACGIH) has assigned carbon monoxide a threshold limit value (TLV) of 25 ppm (29 mg/m(3)) as a TWA for a normal 8-hour workday and a 40-hour workweek [ACGIH 1994, p. 15]. The ACGIH limit is based on the risk of elevated carboxyhemoglobin levels [ACGIH 1991, p. 229].
This guideline highlights important information regarding carbon monoxide for workers, employers, physicians, industrial hygienists, and other occupational safety and health experts who may require it to run effective programs.
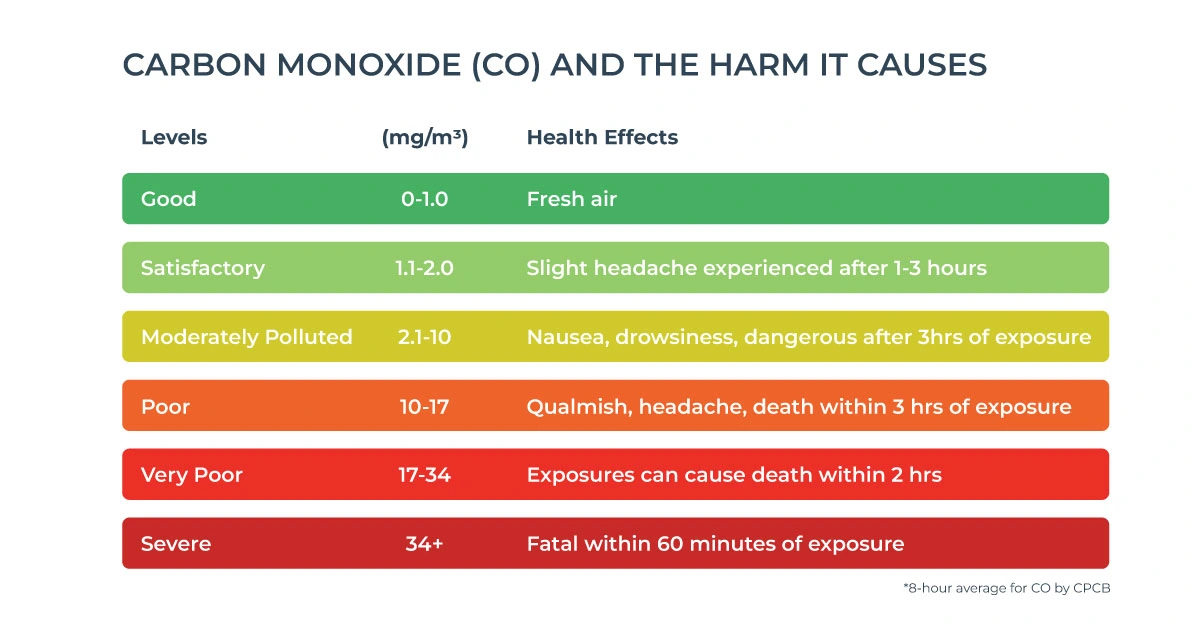
4. Health & Environmental Impact of Carbon Monoxide
Health Impact
CO levels in the atmosphere are generally normal and unlikely to cause harm. When these levels grow in an indoor or outdoor context, a variety of health concerns to both individuals and the environment emerge.
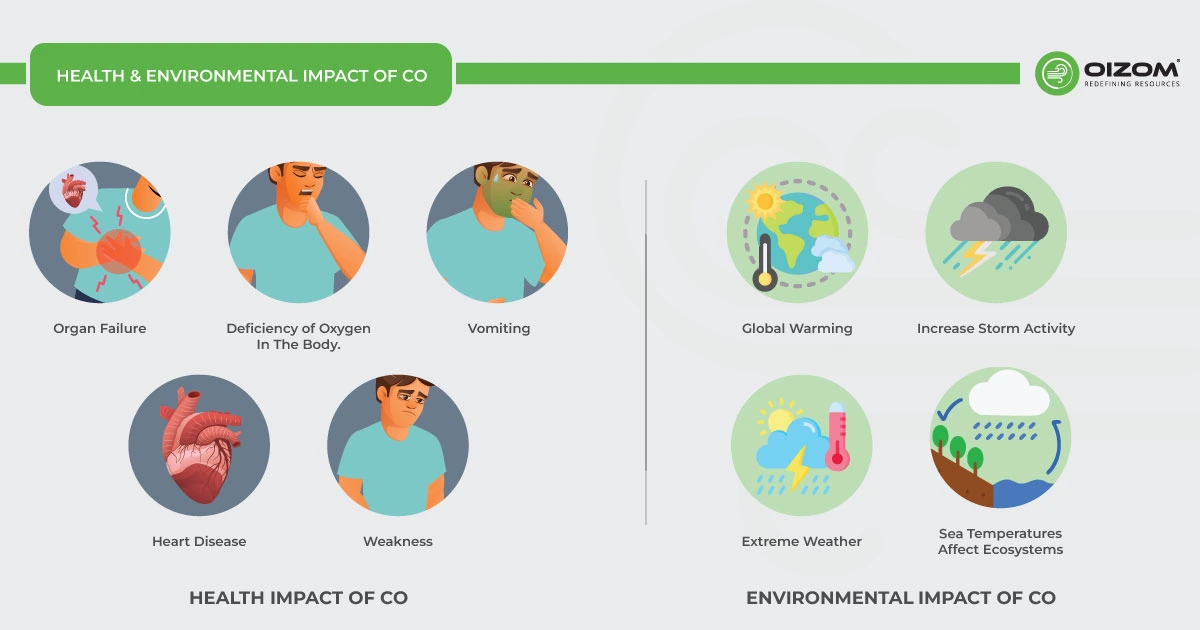
- Many people know carbon monoxide (CO) as a dangerous gas found in homes, but it’s also a common air pollutant. It’s one of the most harmful gases for our health because CO binds to hemoglobin in our blood much more strongly than oxygen, about 200 times more. This prevents oxygen from getting to our organs, which can lead to serious issues like organ failure.
- It is especially harmful to heart patients since it produces a deficiency of oxygen in the body. It makes us feel ill, and one of the most common symptoms is vomiting. CO poisoning can also induce influenza. If not treated immediately, it might cause unconsciousness and, in rare cases, death due to the severe consequences on the body.
- Initial symptoms of CO poisoning may include flu-like symptoms such as headache, dizziness, weakness, nausea, and fatigue. In case of prolonged or high exposures, this may lead to vomiting, loss of consciousness, and collapse. It may lead to coma or death if high exposures continue. People with coronary heart disease may suffer from chest pain.
Did you know this? According to the Centers for Disease Control, Each year, more than 400 Americans die from unintentional CO poisoning not linked to fires, more than 100,000 visit the emergency room, and more than 14,000 are hospitalized.
Environmental Impact
Carbon monoxide affects the amount of greenhouse gases emitted into the environment. This alteration in the atmosphere is linked to climate change and global warming, as rising land and sea temperatures affect ecosystems, increase storm activity, and cause other extreme weather events.
On a worldwide scale, CO has no substantial environmental impact. However, CO can react with other air contaminants at the emission source. During the summer, it can generate hazardous ground-level ozone or oxidize to form carbon dioxide (CO2). This important greenhouse gas aids in the trapping of heat in the atmosphere. CO is classified as a short-lived climate forcing agent, prompting CO emission reductions to be considered a possible strategy to mitigate the effects of global warming.
The reactions of CO in the atmosphere leading to hydroxyl conversion and ozone formation are:
CO + OH ➝ CO2 + H
H + O2 ➝ HO2
HO2 + NO ➝ OH + NO2
NO2 + sunlight ➝ NO + O
O + O2 ➝ O3
Net: CO + 2O2 ➝ CO2 + O3
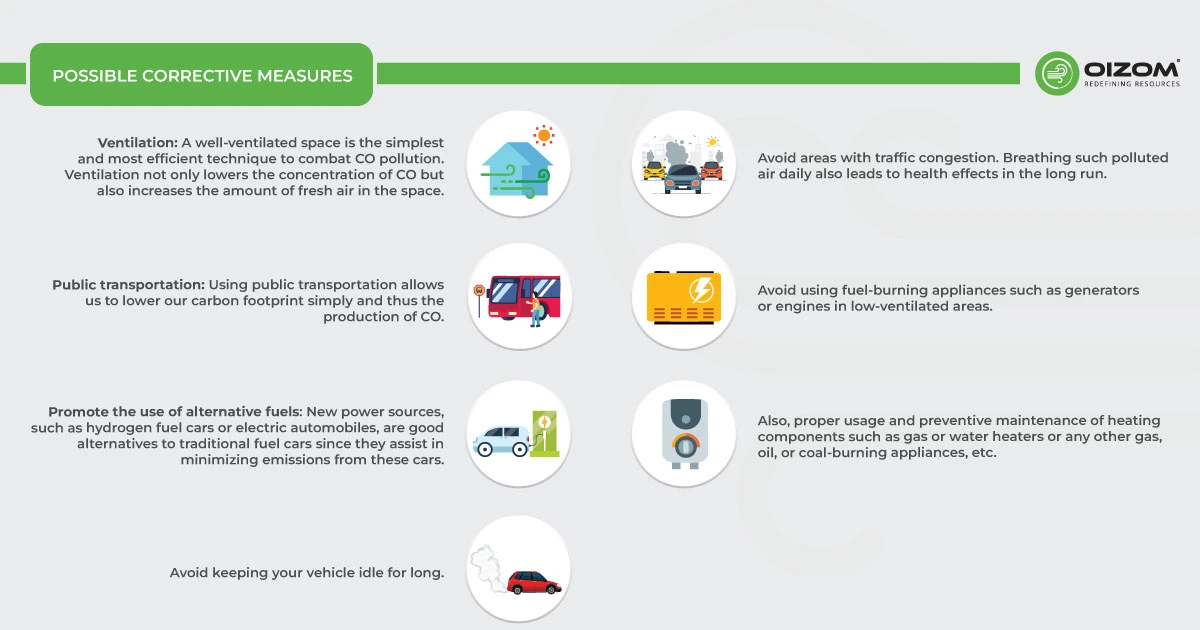
5. Possible Corrective Measures
CO cannot be easily removed using air purifiers or other methods; thus, corrective steps are the only way to avoid exposure in real life. The only way to detect and prevent problems is to monitor them. To reduce exposure and the creation of this harmful gas, preventive measures must be implemented. Some of the measures are mentioned below:
- Ventilation: A well-ventilated space is the simplest and most efficient technique to combat CO pollution. Ventilation not only lowers the concentration of CO but also increases the amount of fresh air in the space.
- Public transportation: Using public transportation allows us to lower our carbon footprint simply and thus the production of CO.
- Promote the use of alternative fuels: New power sources, such as hydrogen fuel cars or electric automobiles, are good alternatives to traditional fuel cars since they assist in minimizing emissions from these cars.
- Avoid keeping your vehicle idle for long.
- Avoid areas with traffic congestion. Breathing such polluted air daily also leads to health effects in the long run.
- Avoid using fuel-burning appliances such as generators or engines in low-ventilated areas.
- Also, proper usage and preventive maintenance of heating components such as gas or water heaters or any other gas, oil, or coal-burning appliances, etc.
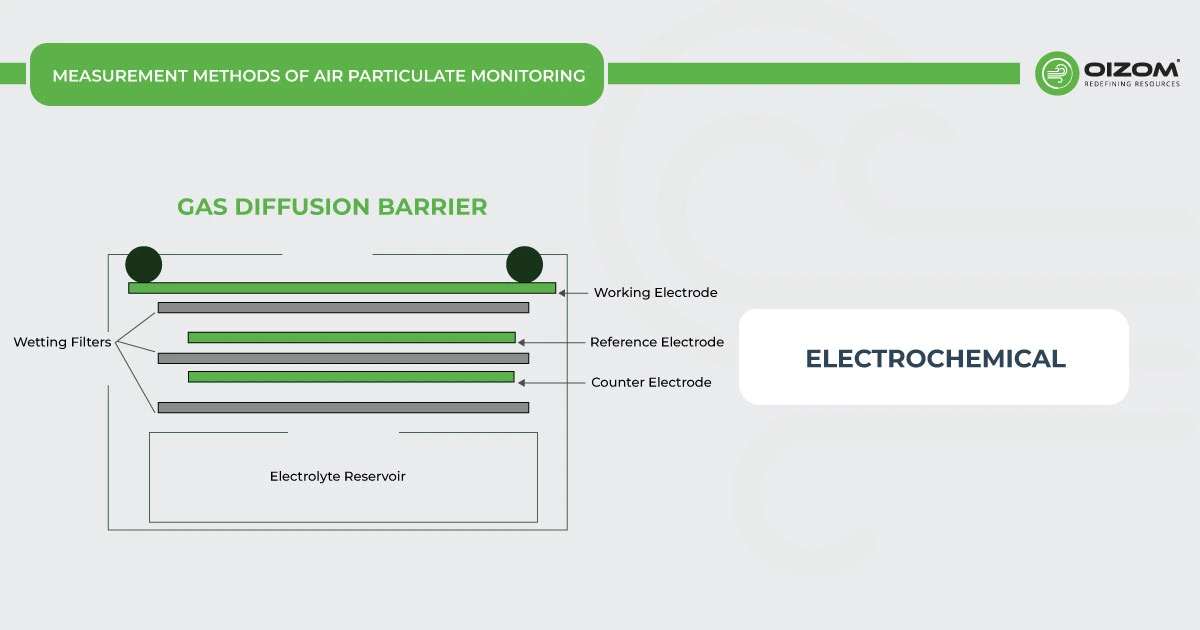
6. Measurement Methods of CO Monitoring
Different working principles for carbon monoxide monitoring in the ambient environment are nondispersive infrared absorption (NDIR), semiconductor, and electrochemistry.
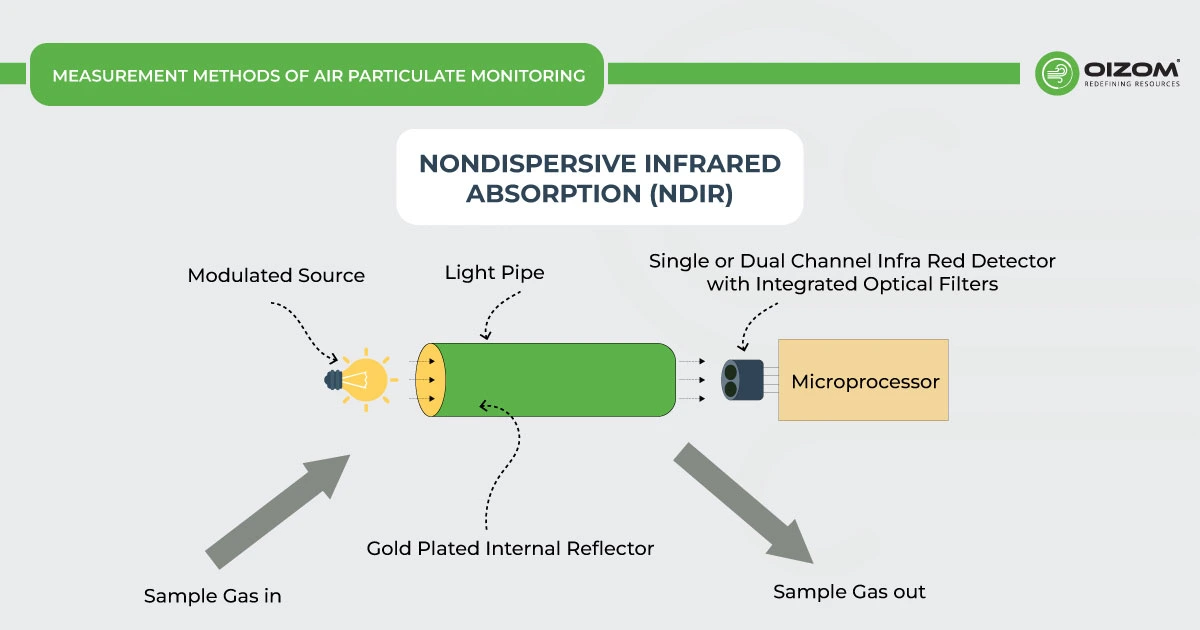
Nondispersive infrared absorption (NDIR) – Carbon monoxide absorbs infrared radiation at a particular frequency. When the gas sample is exposed to infrared radiation, the infrared radiation absorbed by the CO molecules present in the gas sample is measured by the detector in a non-dispersive photometer. However, the use of this principle in carbon monoxide monitors may result in the possibility of potential interferences from other gases that absorb infrared radiation similar to CO.
Semiconductor – When a metal oxide semiconductor-based carbon monoxide monitor is exposed to an air sample, the CO molecules react on the metal oxide surface of the sensor and dissociate into charged ions which alter the resistance of the film. This interaction is measured as a signal and is converted to the gas concentration. However, the energy consumption of such carbon monoxide monitors is higher compared to other CO monitors.
Electrochemical – CO monitors working on the electrochemical principle are operated based on the diffusion of carbon monoxide gas into the sensor which results in the production of an electrical signal proportional to the CO concentration. It allows accurate measurement of even low concentrations of carbon monoxide, which is essential in carbon monoxide monitoring in ambient conditions.
[Source: https://docs.iscape.smartcitizen.me/Components/Gas%20Pro%20Sensor%20Board/Electrochemical%20Sensors/]
7. Oizom’s Sensor Working Principle for CO Monitoring
Oizom provides a range of Carbon Monoxide (CO) sensor modules to monitor varying CO levels based on your needs. Our sensors accurately measure CO in ambient conditions, detecting concentrations in ppb/ppm. This sensor monitors carbon monoxide in real time. This sensor is integrated into a metal casing and ultra-low-noise support electronics, making it compact and reliable. This allows accurate gas detection even at very low concentrations in the atmosphere. This sensor works on the Electrochemical working principle to measure environmental air quality.
This sensor undergoes calibration using standard gases and tools to ensure high sensitivity and accuracy. Each gas sensor is calibrated with zero and span checks in a controlled lab, following Section 12.2 of the USEPA Quality Assurance Handbook for Air Pollution Measurement Systems Volume II. Calibration is done using NIST-traceable gas standards for reliable performance.
The CO sensor module is integrated into outdoor air quality monitoring systems like Polludrone, AQBot, and Odosense. It is ideal for applications such as campus monitoring, smart cities, industries, wastewater treatment plants, roads, research projects, and environmental impact assessments. By utilizing this sensor module, users can ensure they receive accurate, real-time data on CO levels, aiding in the effective monitoring and management of air quality across diverse environments.
8. Why Choose Oizom CO Sensor?
- Compact: Our sensors are small and easy to install, perfect for use in any space, making them ideal for portable air quality monitoring. The CO sensors come pre-calibrated and can be quickly replaced in just a few minutes by removing and replacing the old sensor with a new one.
- Durable: The CO sensor has a long life of almost two years.
- Energy Efficient: There is no need to be concerned about energy usage because our sensors are not only accurate but also energy efficient. Powered up with just 3.3 to 5V for efficient, reliable performance!
- In-house sensor tech: Oizom applies advanced data processing algorithms to compensate for the effects of temperature and humidity on the sensor output. The algorithm is designed to automatically update based on weather conditions and seasonal changes, removing its influence on the sensor performance. The advanced algorithms also compensate for the effect of the cross-sensitive gas.
- Ultra-Low Noise Electronics: The sensor module’s design is crucial for accurate measurements. Each sensor is housed in a metal casing with ultra-low-noise electronics and a base PCB. The metal casing shields the sensor and electronics from electromagnetic interference, preventing false readings.
- RoHS Compliant: Our sensors comply with the RoHS criteria for restricting hazardous substances in electrical and electronic devices.
9. Reasons Why CO Monitoring is Important:
- Carbon monoxide can be released by almost any combustion source, such as vehicles, gas-burning appliances such as gas heaters, generators, furnaces in enclosed places, and many common sources present everywhere, adding to the carbon monoxide levels already in the atmosphere.
- CO cannot be smelled or tasted and cannot be seen, so it remains undetectable by humans even at higher levels, making it a “silent killer.”
- If there is too much carbon monoxide in the air, it can damage your facilities, equipment, and products. Excess CO can accelerate corrosion in high temperatures by a chemical process known as metal dusting, reducing the lifespan of pipelines, fans, automobiles, and other metal equipment. High quantities of carbon monoxide can also interfere with delicate instruments such as sensors and electronics, compromising the quality of your products.
- CO monitoring is an efficient way to prevent CO poisoning. It helps detect the buildup of carbon monoxide levels and alerts when a certain level is exceeded.
- Real-time carbon monoxide monitoring helps calculate the air quality index to deliver health advisories and formulate an action plan to meet standards.
- Due to state, county, and local legislation, carbon monoxide monitoring devices are now required in most workplaces. Even in states without explicit mandates, countries and towns frequently have air quality regulations.
FAQs
- What is carbon monoxide (CO)?
Carbon monoxide (CO) is a colorless, odourless gas produced by the incomplete burning of fuels like gasoline, wood, or natural gas. - How does carbon monoxide affect human health?
CO binds to hemoglobin in the blood, reducing oxygen flow to organs. This can cause symptoms like headaches, dizziness, or even organ failure with high exposure. - Where does carbon monoxide come from?
CO is emitted from sources like vehicle exhaust, industrial activities, gas appliances, and burning wood or coal. - Can carbon monoxide contribute to climate change?
While CO doesn’t directly cause global warming, it affects greenhouse gases and can worsen air pollution, indirectly contributing to climate change.
- How can we reduce carbon monoxide pollution?
Using cleaner energy sources, improving vehicle emissions, and ensuring proper home ventilation can help reduce CO emissions.Oizom7eagles1234
Carbon monoxide (CO) is a gas you can’t see, smell, or taste, but it can be dangerous for us and the environment. It’s produced when things like gasoline or wood don’t burn completely, especially in places with a lot of traffic or industrial activity. Exposure to high levels of CO can cause CO poisoning, leading to serious health issues, giving it the name “The Silent Killer.” While CO is not the primary cause of global warming, it does contribute to disrupting the balance of greenhouse gases, potentially exacerbating climate change. Even small levels of CO can produce headaches and dizziness in humans, and prolonged exposure can be quite hazardous.
Monitoring and reducing CO emissions is essential for creating better, safer environments and tackling broader climate challenges. This article covers information on carbon monoxide, its sources, permissible levels in the ambient air, health and environmental impact, possible corrective measures, the need for carbon monoxide monitors, and different methods of CO monitoring.
1. What is Carbon Monoxide?
Carbon monoxide is a combustible gas without color, taste, or odour. It is the most common and harmful gas found in both indoor and outdoor environments. It is produced by the incomplete combustion of carbon-containing fuels such as natural gas, gasoline, or wood. It is emitted by various combustion sources, including automobiles, power plants, wildfires, and incinerators. Mobile sources account for the vast majority of outdoor CO emissions to ambient air nationally and in metropolitan regions.
Carbon monoxide can also be produced by photochemical reactions involving methane and non-methane hydrocarbons, other volatile organic hydrocarbons in the atmosphere, and organic molecules in surface waters and soil. Some indoor sources of CO also add to overall exposure. The molecular weight of carbon monoxide is similar to that of air: about 28.01 g/mole. Carbon monoxide consists of one oxygen atom and one carbon atom, which are further attached together by a triple bond consisting of two covalent bonds and one dative covalent bond. The chemical formula of carbon monoxide is given as CO. It is flammable and reacts vigorously with oxygen, acetylene, chlorine, fluorine, and nitrous oxide. It is slightly soluble in water, blood serum, and plasma.
Carbon monoxide in Atmosphere:
CO is naturally present in the atmosphere at very low concentrations of about 0.2 ppm, which is not harmful to humans independently. CO is released naturally from volcanic eruptions, forest fires, etc. However, human activities are the biggest source of CO emissions, among which the largest proportion are emitted by the exhaust of internal combustion engines of motor vehicles running on petrol and diesel. It remains in the atmosphere for about two months and eventually reacts with oxygen to form carbon dioxide.
Carbon monoxide is a trace gas in the atmosphere that has no direct effect on global temperature, unlike methane and carbon dioxide. On the other hand, it plays an important part in atmospheric chemistry and impacts the atmosphere’s ability to cleanse itself of many other pollutants. In combination with other pollutants and sunlight, it contributes to the development of lower-atmospheric (“bad”) ozone and urban smog.
The levels of carbon monoxide are typically highest during winters as cold temperatures inhibit the combustion processes, resulting in higher CO formation. Additionally, the meteorological conditions during winters trap the pollutants near the ground. The use of gas furnaces and heaters increases in this season, adding to CO emissions.
2. Sources
Carbon monoxide (CO) is a dangerous gas that can be inhaled in large quantities. It can be released from a variety of anthropogenic sources and also exists naturally.
Natural
Carbon monoxide is emitted from natural sources such as volcanoes, bush/forest fires, natural gases released from coal mines, the metabolism of animals, and even lightning.
Anthropogenic
- Combustion occurs in commercial vehicles such as cars, airplanes, tractors, and trucks.
- Industries working at high temperatures and using carbon compounds as raw products.
- Additional sources include breathing in tobacco smoke, burning biomass for cooking, equipment, or appliances such as heaters, barbeques, gas stoves, gas water heaters, fireplaces, Extracting gas from sea or land, etc.
3. Permissible levels of Carbon Monoxide
The breakpoint concentrations describing air quality based on the carbon monoxide concentrations for different countries are given below.
Table: Breakpoints of carbon monoxide (mg/m3)
|
India (8-hr) |
US (24-hr) |
China (24-hr) |
EU (8-hr) |
||||
|
AQI Category |
Breakpoint concentration |
AQI Category |
Breakpoint concentration |
AQI Category |
Breakpoint concentration |
AQI Category |
Breakpoint concentration |
|
Good |
1 |
Good |
5 |
Excellent |
2 |
Very low |
5 |
|
Satisfactory |
2 |
Moderate |
11 |
Good |
4 |
Low |
7.5 |
|
Moderately polluted |
10 |
Unhealthy for sensitive |
14 |
Lightly Polluted |
14 |
Medium |
10 |
|
Poor |
17 |
Unhealthy |
18 |
Moderately Polluted |
24 |
High |
20 |
|
Very Poor |
34 |
Very Unhealthy |
35 |
Heavily Polluted |
36 |
Very high |
20+ |
|
Severe |
34+ |
Hazardous |
58 |
Severely Polluted |
36+ |
||
- OSHA PEL: The current Occupational Safety and Health Administration (OSHA) permissible exposure limit (PEL) for carbon monoxide is 50 parts per million (ppm) parts of air (55 milligrams per cubic meter (mg/m(3))) as an 8-hour time-weighted average (TWA) concentration [29 CFR Table Z-1].
- NIOSH REL: The National Institute for Occupational Safety and Health (NIOSH) has established a recommended exposure limit (REL) for carbon monoxide of 35 ppm (40 mg/m(3)) as an 8-hour TWA and 200 ppm (229 mg/m(3)) as a ceiling [NIOSH 1992]. The NIOSH limit is based on the risk of cardiovascular effects.
- ACGIH TLV: The American Conference of Governmental Industrial Hygienists (ACGIH) has assigned carbon monoxide a threshold limit value (TLV) of 25 ppm (29 mg/m(3)) as a TWA for a normal 8-hour workday and a 40-hour workweek [ACGIH 1994, p. 15]. The ACGIH limit is based on the risk of elevated carboxyhemoglobin levels [ACGIH 1991, p. 229].
This guideline highlights important information regarding carbon monoxide for workers, employers, physicians, industrial hygienists, and other occupational safety and health experts who may require it to run effective programs.
4. Health & Environmental Impact of Carbon Monoxide
Health Impact
CO levels in the atmosphere are generally normal and unlikely to cause harm. When these levels grow in an indoor or outdoor context, a variety of health concerns to both individuals and the environment emerge.
- Many people know carbon monoxide (CO) as a dangerous gas found in homes, but it’s also a common air pollutant. It’s one of the most harmful gases for our health because CO binds to hemoglobin in our blood much more strongly than oxygen, about 200 times more. This prevents oxygen from getting to our organs, which can lead to serious issues like organ failure.
- It is especially harmful to heart patients since it produces a deficiency of oxygen in the body. It makes us feel ill, and one of the most common symptoms is vomiting. CO poisoning can also induce influenza. If not treated immediately, it might cause unconsciousness and, in rare cases, death due to the severe consequences on the body.
- Initial symptoms of CO poisoning may include flu-like symptoms such as headache, dizziness, weakness, nausea, and fatigue. In case of prolonged or high exposures, this may lead to vomiting, loss of consciousness, and collapse. It may lead to coma or death if high exposures continue. People with coronary heart disease may suffer from chest pain.
Did you know this? According to the Centers for Disease Control, Each year, more than 400 Americans die from unintentional CO poisoning not linked to fires, more than 100,000 visit the emergency room, and more than 14,000 are hospitalized.
Environmental Impact
Carbon monoxide affects the amount of greenhouse gases emitted into the environment. This alteration in the atmosphere is linked to climate change and global warming, as rising land and sea temperatures affect ecosystems, increase storm activity, and cause other extreme weather events.
On a worldwide scale, CO has no substantial environmental impact. However, CO can react with other air contaminants at the emission source. During the summer, it can generate hazardous ground-level ozone or oxidize to form carbon dioxide (CO2). This important greenhouse gas aids in the trapping of heat in the atmosphere. CO is classified as a short-lived climate forcing agent, prompting CO emission reductions to be considered a possible strategy to mitigate the effects of global warming.
The reactions of CO in the atmosphere leading to hydroxyl conversion and ozone formation are:
CO + OH ➝ CO2 + H
H + O2 ➝ HO2
HO2 + NO ➝ OH + NO2
NO2 + sunlight ➝ NO + O
O + O2 ➝ O3
Net: CO + 2O2 ➝ CO2 + O3
5. Possible Corrective Measures
CO cannot be easily removed using air purifiers or other methods; thus, corrective steps are the only way to avoid exposure in real life. The only way to detect and prevent problems is to monitor them. To reduce exposure and the creation of this harmful gas, preventive measures must be implemented. Some of the measures are mentioned below:
- Ventilation: A well-ventilated space is the simplest and most efficient technique to combat CO pollution. Ventilation not only lowers the concentration of CO but also increases the amount of fresh air in the space.
- Public transportation: Using public transportation allows us to lower our carbon footprint simply and thus the production of CO.
- Promote the use of alternative fuels: New power sources, such as hydrogen fuel cars or electric automobiles, are good alternatives to traditional fuel cars since they assist in minimizing emissions from these cars.
- Avoid keeping your vehicle idle for long.
- Avoid areas with traffic congestion. Breathing such polluted air daily also leads to health effects in the long run.
- Avoid using fuel-burning appliances such as generators or engines in low-ventilated areas.
- Also, proper usage and preventive maintenance of heating components such as gas or water heaters or any other gas, oil, or coal-burning appliances, etc.
6. Measurement Methods of CO Monitoring
Different working principles for carbon monoxide monitoring in the ambient environment are nondispersive infrared absorption (NDIR), semiconductor, and electrochemistry.
Nondispersive Infrared Absorption (NDIR) – Carbon monoxide absorbs infrared radiation at a particular frequency. When the gas sample is exposed to infrared radiation, the infrared radiation absorbed by the CO molecules present in the gas sample is measured by the detector in a non-dispersive photometer. However, the use of this principle in carbon monoxide monitors may result in the possibility of potential interferences from other gases that absorb infrared radiation similar to CO.
Semiconductor – When a metal oxide semiconductor-based carbon monoxide monitor is exposed to an air sample, the CO molecules react on the metal oxide surface of the sensor and dissociate into charged ions which alter the resistance of the film. This interaction is measured as a signal and is converted to the gas concentration. However, the energy consumption of such carbon monoxide monitors is higher compared to other CO monitors.
Electrochemical – CO monitors working on the electrochemical principle are operated based on the diffusion of carbon monoxide gas into the sensor which results in the production of an electrical signal proportional to the CO concentration. It allows accurate measurement of even low concentrations of carbon monoxide, which is essential in carbon monoxide monitoring in ambient conditions.
[Source: https://docs.smartcitizen.me/Components/sensors/Electrochemical%20Sensors/]
7. Oizom’s Sensor Working Principle for CO monitoring
Oizom provides a range of Carbon Monoxide (CO) sensor modules to monitor varying CO levels based on your needs. Our sensors accurately measure CO in ambient conditions, detecting concentrations in ppb/ppm. This sensor monitors carbon monoxide in real time. This sensor is integrated into a metal casing and ultra-low-noise support electronics, making it compact and reliable. This allows accurate gas detection even at very low concentrations in the atmosphere. This sensor works on the Electrochemical working principle to measure environmental air quality.
This sensor undergoes calibration using standard gases and tools to ensure high sensitivity and accuracy. Each gas sensor is calibrated with zero and span checks in a controlled lab, following Section 12.2 of the USEPA Quality Assurance Handbook for Air Pollution Measurement Systems Volume II. Calibration is done using NIST-traceable gas standards for reliable performance.
The CO sensor module is integrated into outdoor air quality monitoring systems like Polludrone, AQBot, and Odosense. It is ideal for applications such as campus monitoring, smart cities, industries, wastewater treatment plants, roads, research projects, and environmental impact assessments. By utilizing this sensor module, users can ensure they receive accurate, real-time data on CO levels, aiding in the effective monitoring and management of air quality across diverse environments.
8. Why Choose Oizom CO Sensor?
- Compact: Our sensors are small and easy to install, perfect for use in any space, making them ideal for portable air quality monitoring. The CO sensors come pre-calibrated and can be quickly replaced in just a few minutes by removing and replacing the old sensor with a new one.
- Durable: The CO sensor has a long life of almost two years.
- Energy Efficient: There is no need to be concerned about energy usage because our sensors are not only accurate but also energy efficient. Powered up with just 3.3 to 5V for efficient, reliable performance!
- In-house sensor tech: Oizom applies advanced data processing algorithms to compensate for the effects of temperature and humidity on the sensor output. The algorithm is designed to automatically update based on weather conditions and seasonal changes, removing its influence on the sensor performance. The advanced algorithms also compensate for the effect of the cross-sensitive gas.
- Ultra-Low Noise Electronics: The sensor module’s design is crucial for accurate measurements. Each sensor is housed in a metal casing with ultra-low-noise electronics and a base PCB. The metal casing shields the sensor and electronics from electromagnetic interference, preventing false readings.
- RoHS Compliant: Our sensors comply with the RoHS criteria for restricting hazardous substances in electrical and electronic devices.
9. Reasons Why CO Monitoring is Important:
- Carbon monoxide can be released by almost any combustion source, such as vehicles, gas-burning appliances such as gas heaters, generators, furnaces in enclosed places, and many common sources present everywhere, adding to the carbon monoxide levels already in the atmosphere.
- CO cannot be smelled or tasted and cannot be seen, so it remains undetectable by humans even at higher levels, making it a “silent killer.”
- If there is too much carbon monoxide in the air, it can damage your facilities, equipment, and products. Excess CO can accelerate corrosion in high temperatures by a chemical process known as metal dusting, reducing the lifespan of pipelines, fans, automobiles, and other metal equipment. High quantities of carbon monoxide can also interfere with delicate instruments such as sensors and electronics, compromising the quality of your products.
- CO monitoring is an efficient way to prevent CO poisoning. It helps detect the buildup of carbon monoxide levels and alerts when a certain level is exceeded.
- Real-time carbon monoxide monitoring helps calculate the air quality index to deliver health advisories and formulate an action plan to meet standards.
- Due to state, county, and local legislation, carbon monoxide monitoring devices are now required in most workplaces. Even in states without explicit mandates, countries and towns frequently have air quality regulations.
FAQs
- What is carbon monoxide (CO)?
Carbon monoxide (CO) is a colorless, odourless gas produced by the incomplete burning of fuels like gasoline, wood, or natural gas. - How does carbon monoxide affect human health?
CO binds to hemoglobin in the blood, reducing oxygen flow to organs. This can cause symptoms like headaches, dizziness, or even organ failure with high exposure. - Where does carbon monoxide come from?
CO is emitted from sources like vehicle exhaust, industrial activities, gas appliances, and burning wood or coal. - Can carbon monoxide contribute to climate change?
While CO doesn’t directly cause global warming, it affects greenhouse gases and can worsen air pollution, indirectly contributing to climate change.
- How can we reduce carbon monoxide pollution?
Using cleaner energy sources, improving vehicle emissions, and ensuring proper home ventilation can help reduce CO emissions.