Formaldehyde (CH2O), also known as methanal, is a colorless gas with a strong, noticeable smell (pickle-like pungent odour) found indoors and outdoors. It is released from emissions of almost every volatile organic hydrocarbon. It’s commonly used in industries for making things like building materials, furniture, and household products. But when it comes to the environment and our health, formaldehyde can be a real concern. Indoors, it’s often released from items like pressed-wood furniture, paints, and adhesives, which can lower the air quality in homes, schools, and workplaces.
Formaldehyde doesn’t just go away in the environment. It is released through natural processes, reactions involving volatile organic compounds in the air, and the burning of fuels or biomass. This affects the air we breathe, whether we’re in the city or the countryside. While it might not seem harmful at first, even low exposure over time can cause problems like eye, nose, and throat irritation, and in more severe cases, it can lead to respiratory issues or even cancer. Using formaldehyde monitors is a viable solution to provide adequate warning of hazardous exposure. This article covers information on formaldehyde, its sources in the ambient air, permissible levels, health and environmental impact, possible corrective measures, the need for formaldehyde monitors, and different methods of CH2O monitoring.
What is CH2O?
Formaldehyde is the simplest aldehyde. It is made of hydrogen, carbon, and oxygen and has the formula CH2O (or H−CHO). Formaldehyde belongs to a broad group of substances called volatile organic compounds, which, at normal temperatures, evaporate and turn into gaseous forms. Its pure gaseous form polymerizes spontaneously into paraformaldehyde; hence, it is usually stored in its liquid form, formalin.
The World Health Organization (WHO) classified formaldehyde (CH2O) as a Group 1 human carcinogen in 2004. The European Commission categorized formaldehyde as a 1B carcinogen and mutagen in 2014. Formaldehyde is produced by natural processes and was first synthesized in 1867; its annual global production currently exceeds 30 million tons. {Ref. Outdoor formaldehyde matters and substantially impacts indoor formaldehyde concentrations, Building and Environment, Volume 158, July 2019, pp. 145-150}
The main chemical and physical properties of CH2O are as follows :
- Molecular weight/molar mass = 30.03 g/mol
- Relative vapor density = 1.03–1.07 (air = 1)
- Density = 815 kg/m³
- Melting point = −92 °C and boiling point = −19.1 °C.
- Soluble in water (around 400 g/l at 20 °C), ethanol, chloroform, and miscible with acetone, benzene, and diethyl ether.
Formaldehyde dissolves easily in water (known as formalin) but does not last long there, either. Formalin is commonly used as an industrial disinfectant and preservative in funeral homes and medical labs. When an item emits formaldehyde, it is released into the air through a process called off-gassing. Formaldehyde gas is quickly broken down in the air, generally within hours.
Formaldehyde in Atmosphere
Formaldehyde is the most common aldehyde in the environment. The natural background concentration is < 1 µg/m3 with a mean of about 0.5 µg/m3. [Ref– Formaldehyde. In: Wood dust and formaldehyde. Lyon, International Agency for Research on Cancer, 1995, pp. 217–362.]
Being a ubiquitous trace chemical in the ambient atmosphere, formaldehyde (CH2O) plays an important role in atmospheric photochemistry because it reacts quickly in the atmosphere, and its photolysis provides new hydroxyl (OH) and hydroperoxy radicals (HO2). CH2O can also influence secondary organic aerosol (SOA) by providing radicals that increase gas-phase oxidation of hydrocarbons and increase surface-active organic material. When produced in the atmosphere by the action of sunlight and oxygen on atmospheric methane and other hydrocarbons, it becomes part of smog. Formaldehyde does not accumulate in the environment because it is broken down within a few hours by sunlight or bacteria in soil or water.
2. Sources
Natural Source
Formaldehyde occurs naturally in the environment. It is produced in the atmosphere from emissions of almost every volatile organic hydrocarbon. It is also produced during the decay of plant material in the soil and normal chemical processes in most living organisms. It is also a combustion product found in tobacco smoke. Humans and most other living organisms make small amounts as part of normal metabolic processes.
In addition, formaldehyde is a byproduct of all combustion processes, including cooking, vehicle exhaust, and forest fires. Bananas, apples, carrots, and other fruits and vegetables all naturally contain low amounts of formaldehyde.
Did you know? Formaldehyde naturally occurs in many food products, with levels varying widely. For example, milk contains about 0.1-0.3 mg/kg, while some fish species can have over 200 mg/kg. Many fruits also produce formaldehyde naturally, including bananas (16.3 mg/kg), pears (38.7 mg/kg), grapes (22.4 mg/kg), and apples (22.3 mg/kg).
Anthropogenic Sources
Incomplete combustion (power plants, incineration, etc.) and other oxidation processes of longer-chain organic molecules, such as methane and volatile organic compounds (VOCs), can also produce formaldehyde. Smoking also produces formaldehyde.
The photooxidation of methane in the environment produces formaldehyde, which is present in trace amounts in the atmosphere, roughly one part per billion. Formaldehyde is commonly used in construction materials, insulation, glues, permanent press fabrics, paints, lacquers, and other coatings. It is also present in composite wood products that incorporate formaldehyde-containing resins.
Personal care goods that contain formaldehyde, such as body washes, nail polish, shampoos, soaps, and hair care products, also emit formaldehyde into the atmosphere. Furthermore, many other consumer goods release volatile organic compounds (VOCs), which combine with atmospheric ozone to formaldehyde.
Were you aware of this? Humans naturally produce about 1.5 ounces of formaldehyde daily as part of normal metabolism. We also inhale around 0.0007 ounces when exposed to levels that match the World Health Organization (WHO) Indoor Air Quality Guideline. Once inhaled, formaldehyde is quickly broken down into carbon dioxide and exhaled.
3. Permissible levels of Formaldehyde
The permissible exposure limits of CH2O in terms of continuous occupational exposure are given below:
|
TWA |
STEL |
Ceiling limit |
IDHL |
|
|
OSHA PEL |
0.75 ppm (8-hr) |
2 ppm |
– |
– |
|
NIOSH REL |
0.016 ppm (10-hr) |
– |
0.1 ppm (15 mins) |
20 ppm |
|
ACGIH TLV |
0.1 ppm (8-hr) |
0.3 ppm |
– |
– |
The above exposure limits are for air levels only. When skin contact also occurs, you may be overexposed, even though air levels are less than the limits listed above.
Permissible Exposure Limit (PEL) given by OSHA (Occupational Safety and Health Administration) defines the maximum concentration of CH2O to which an unprotected worker may be exposed. PEL may reference an eight-hour time-weighted average (TWA), a 15-minute short-term exposure limit (STEL), or an instantaneous ceiling limit (CL) concentration that cannot be exceeded for any period of time. Similarly, the Recommended Exposure Limit (REL) is the occupational exposure limit recommended by NIOSH (National Institute for Occupational Safety and Health), and the TLVs (i.e., threshold limit values) are the exposure guidelines given by ACGIH (American Conference of Governmental Industrial Hygienists)
4. Health and Environmental Impacts of Formaldehyde
Health Impact
Formaldehyde causes cancer. Evidence shows formaldehyde can cause a rare cancer of the nasopharynx, which is the upper part of the throat behind the nose.
Formaldehyde causes eye, throat, and nasal irritation. Low concentrations of formaldehyde can cause these irritations. Other short-term side effects include breathing difficulties, headaches, runny noses, and nausea. Wheezing, asthma episodes, and other respiratory problems may result after exposure.
Formaldehyde transforms methanol into formic acid, which destroys retinal proteins very quickly. This can result in blindness. Additionally, some people are more sensitive to chemicals such as formaldehyde and may experience symptoms earlier than others.
|
CH2O Concentration (ppm) |
Effects on Health |
|
0.5 |
Slight eye irritation |
|
1 |
Irritation to the eyes and upper breathing tracts (nose, throat) |
|
4 – 5 |
Irritation of trachea and bronchi |
|
10 |
Important Breathing Disturbance |
|
20 |
Risk of Severe Pulmonary edema |
|
50 |
Risk of Severe Damage to tracts |
|
150 |
Lethal within minutes |
Environmental
Formaldehyde also causes harmful effects on the environment and biodiversity.
- On air quality: It is a volatile organic molecule (VOC) that combines with photochemical processes to generate tropospheric ozone, or smog, which harms human health.
- On water and soils: Formaldehyde can be carried by water and end up in lakes, rivers, and soils, where it can have a negative impact on ecosystems on land and in the water.
- On biodiversity: Overexposure to formaldehyde emissions can harm biodiversity by upsetting ecosystems and natural habitats (e.g., slowing down the growth of trees or producing hemorrhages in birds’ lungs).
- On climate change: Formaldehyde doesn’t directly cause climate change, but it can indirectly do so by interacting with other gases in the atmosphere to create compounds that do.
5. Possible Corrective Measures
The primary action is formaldehyde monitoring, i.e., measuring the CH2O concentrations to which you are exposed. It is critical to take action to reduce formaldehyde exposure and minimize emissions. Here are some ways to protect yourself and help lessen the environmental impact of formaldehyde:
In a Company:
- Respect the safety rules: If you operate in a workplace where formaldehyde is utilized, make sure to follow safety protocols, wear appropriate protection equipment, and work in a well-ventilated location.
- Substitute formaldehyde: Wherever possible, consider safer alternatives to formaldehyde in industrial processes.
- Implement emission-reducing strategies: Use environmental monitoring devices and control techniques to reduce formaldehyde emissions into the air.
In the House
- Ventilate regularly: Maintain proper interior ventilation to lower the levels of formaldehyde in the air.
- Avoid goods with formaldehyde: When purchasing furniture or consumer products, look for products that are certified as “low formaldehyde” or “no formaldehyde added”.
- Indoor smoking and vaping are strictly prohibited: Avoiding indoor smoking and vaping can help limit formaldehyde exposure. In addition to formaldehyde, secondhand smoke contains various other compounds that harm one’s health.
- Before you wear permanent press clothing, wash it. Formaldehyde is utilized in the manufacture of specialized fabrics.
6. Measurement Methods of CH2O Monitoring
Different working principles for monitoring formaldehyde in the ambient environment are chemiluminescence, semiconductor, and electrochemistry.
Proton transfer reaction mass spectrometry (PTR-MS) – This method of formaldehyde monitoring uses proton-transfer reactions with H3O+ (hydronium) ions to ionize atmospheric formaldehyde molecules and mass-spectrometric detection of the product ions to know the concentration of CH2O. Based on this principle, the formaldehyde monitor consists of an external ion source, a drift tube, and a mass analysis detection system.
The proton-transfer chemistry occurs in a drift-tube reactor, which enhances the ion kinetic energy and effectively limits cluster ion formation with the abundant water molecules in ambient air. This simplifies both the proton-transfer chemistry and the interpretation of the mass spectra. The exothermicity of the proton-transfer reactions is low enough that the extent of product ion fragmentation is limited, and product ion masses can be used as a unique identifier to detect the formaldehyde concentration. However, the slightly higher proton affinity of formaldehyde than that of water leads to low and strongly humidity-dependent sensitivity of formaldehyde in such formaldehyde monitors.
Semiconductor – When a metal oxide semiconductor-based formaldehyde monitor is exposed to the air sample, the CH2O molecules react on the metal oxide surface of the sensor and dissociate into charged ions, altering the film’s resistance. This interaction is measured as a signal and is converted to the gas concentration. However, the energy consumption of such formaldehyde monitors is higher than that of other monitors.
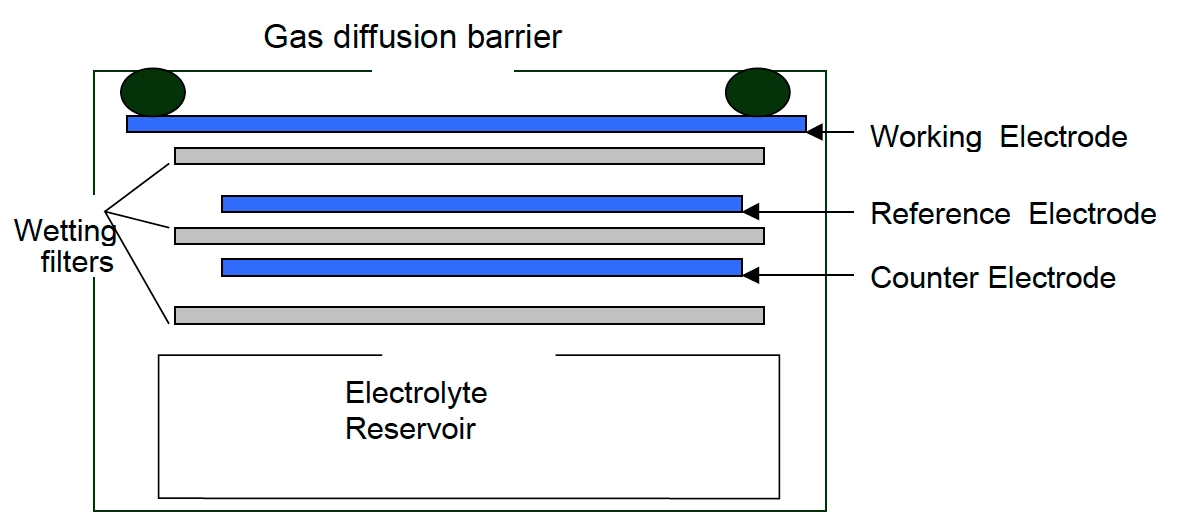
Electrochemical – CH2O monitors working on the electrochemical principle are operated based on the diffusion of formaldehyde gas into the sensor, which results in the production of electrical signals proportional to the CH2O concentration. It allows accurate measurement of even low concentrations of CH2O, which is essential in formaldehyde monitoring in the ambient air.
[Source: iSCAPE Sensors Documentation]
8. Oizom’s Sensor Working Principle for CH2O Monitoring
Oizom provides a range of formaldehyde (CH2O) sensor modules to monitor varying CH2O levels based on your needs. Our sensors accurately measure CH2O in ambient conditions, detecting concentrations in ppb/ppm. This sensor monitors carbon monoxide in real time. This sensor is integrated into a metal casing and ultra-low-noise support electronics, making it compact and reliable. This allows accurate gas detection even at very low concentrations in the atmosphere. This sensor works on the Electrochemical working principle to measure environmental air quality.
This sensor undergoes calibration using standard gases and tools to ensure high sensitivity and accuracy. Each gas sensor is calibrated with zero and span checks in a controlled lab, following Section 12.2 of the USEPA Quality Assurance Handbook for Air Pollution Measurement Systems Volume II. Calibration is done using NIST-traceable gas standards for reliable performance.
The CH2O sensor module is integrated into odour and air quality monitoring systems like AQBot and Odosense. Therefore, this module is ideal for applications like hospitals, medical facilities, Industries, and the manufacturing of wood products such as furniture, resin, fertilizers, pesticides, and oil refining.
8. Why Choose Oizom CH2O Sensor?
- Compact: Our sensors are small and easy to install, perfect for use in any space, making them ideal for portable air quality monitoring. The CH2O sensors come pre-calibrated and can be quickly replaced in just a few minutes by removing and replacing the old sensor with a new one.
- Durable: The CH2O sensor has a long life of almost two years.
- Energy Efficient: There is no need to be concerned about energy usage because our sensors are not only accurate but also energy efficient. Powered up with just 3.3 to 5V for efficient, reliable performance!
- In-house sensor tech: Oizom applies advanced data processing algorithms to compensate for the effects of temperature and humidity on the sensor output. The algorithm is designed to automatically update based on weather conditions and seasonal changes, removing its influence on the sensor performance. The advanced algorithms also compensate for the effect of the cross-sensitive gas.
- Ultra-Low Noise Electronics: The sensor module’s design is crucial for accurate measurements. Each sensor is housed in a metal casing with ultra-low-noise electronics and a base PCB. The metal casing shields the sensor and electronics from electromagnetic interference, preventing false readings.
- RoHS Compliant: Our sensors comply with the RoHS criteria for restricting hazardous substances in electrical and electronic devices.
9. Reasons Why CH2O Monitoring is Important
- Formaldehyde is a colorless gas with a strong pickle-like pungent odour that easily reacts in the atmosphere to form other compounds or gets broken down in soil and water within days of emission.
- Think about any recent changes in your home, like adding new pressed wood items such as cabinets, flooring, or furniture or using coatings or finishes on floors or other surfaces.
- In direct contact with liquid solutions, formaldehyde may cause allergies, respiratory or eye discomfort, and skin irritation. A concentration of 30 mg/m³ poses a life-threatening risk.
- Prolonged exposure to CH2O can quickly deaden a person’s sense of smell, making the odour of methyl mercaptan an unreliable indicator of its presence. Hence, other means, such as using formaldehyde monitors, are viable to provide adequate warning of hazardous exposure.
- It is one of the most common VOCs found in ambient air. Real-time monitoring of CH2O levels helps determine their source and formulate an action plan to control methyl mercaptan emissions.
- Lawmakers, regulators, and businesses have achieved major step in the past several decades in reducing formaldehyde emissions from composite wood products.
FAQs
1. What is Formaldehyde (CH2O)?
Formaldehyde is a colorless gas with a strong odour commonly released from industrial processes and building materials.
2. How does CH2O affect the environment?
CH2O contributes to air pollution and can lead to the formation of ground-level ozone, impacting air quality and ecosystems.
3. Which industries release CH2O into the air?
Industries like manufacturing, wood processing, and chemical production are key sources of formaldehyde emissions.
4. Is CH2O harmful to humans?
Yes, exposure to high levels can cause respiratory irritation and headaches and has been linked to cancer with long-term exposure.
5. How can we control CH2O emissions?
Using air quality monitoring systems and adopting cleaner technologies in industries can help reduce CH2O emissions and protect the environment.
6. Is CH2O soluble in water?
Yes, formaldehyde is highly soluble in water and is sold as an aqueous solution called formalin.
7. Is CH2O a hydrocarbon?
No, CH2O is not a hydrocarbon. It contains oxygen and is classified as an aldehyde.
8.What is the molecular formula for CH2O?
The molecular formula is CH2O, with one carbon, two hydrogen, and one oxygen atom.
9.What happens when ammonia reacts with formaldehyde?
Depending on the reaction conditions, ammonia (NH3) reacts with formaldehyde (CH2O) to form urotropine or methylamino alcohols.